Imara M&A
Review of Asciminib (Scemblix®), 4th Generation Allosteric TKI
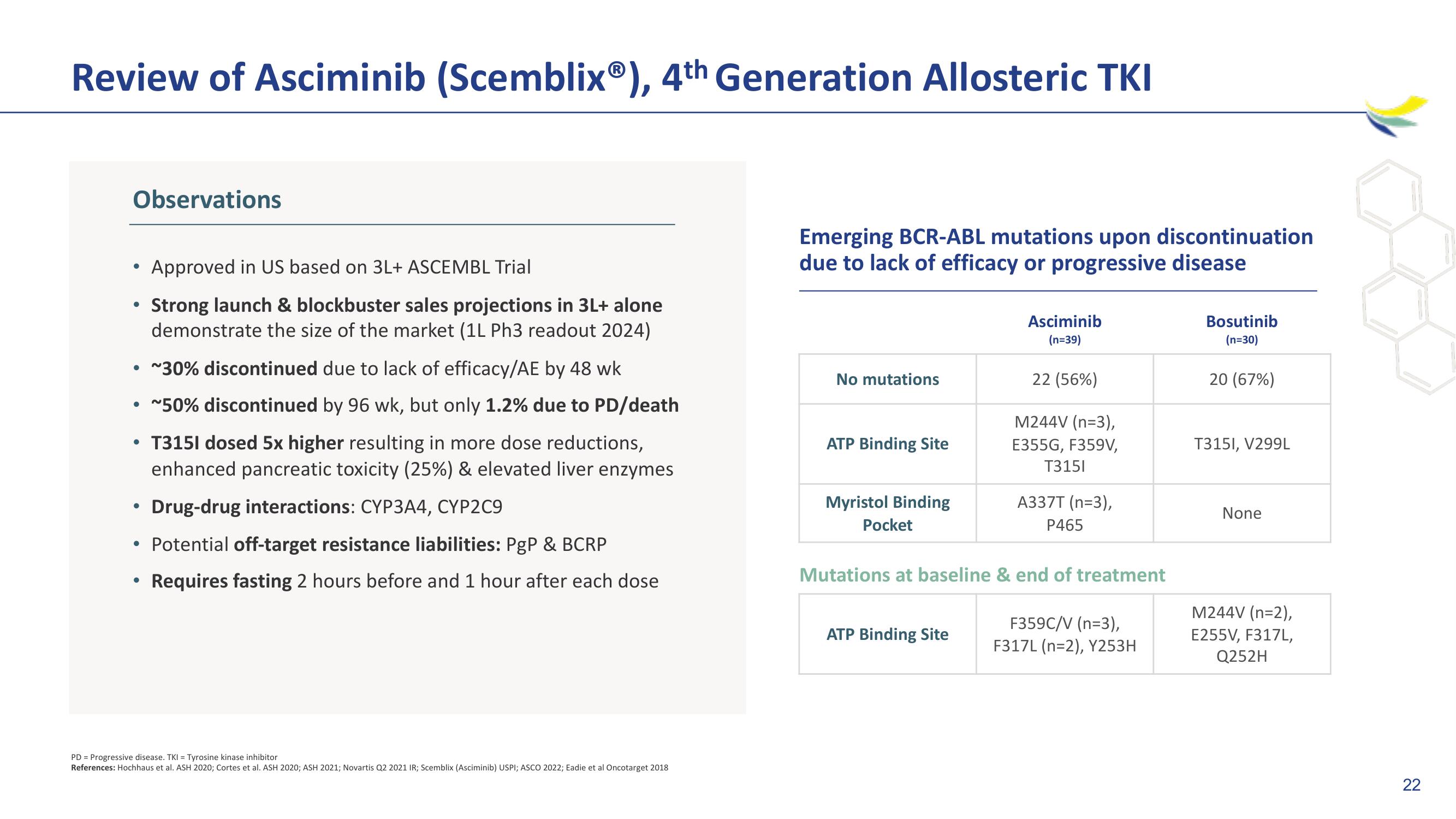
Observations
●
• ~30% discontinued due to lack of efficacy/AE by 48 wk
• ~50% discontinued by 96 wk, but only 1.2% due to PD/death
T3151 dosed 5x higher resulting in more dose reductions,
enhanced pancreatic toxicity (25%) & elevated liver enzymes
Drug-drug interactions: CYP3A4, CYP2C9
Potential off-target resistance liabilities: PgP & BCRP
Requires fasting 2 hours before and 1 hour after each dose
●
●
.
Approved in US based on 3L+ ASCEMBL Trial
Strong launch & blockbuster sales projections in 3L+ alone
demonstrate the size of the market (1L Ph3 readout 2024)
●
PD Progressive disease. TKI = Tyrosine kinase inhibitor
References: Hochhaus et al. ASH 2020; Cortes et al. ASH 2020; ASH 2021; Novartis Q2 2021 IR; Scemblix (Asciminib) USPI; ASCO 2022; Eadie et al Oncotarget 2018
Emerging BCR-ABL mutations upon discontinuation
due to lack of efficacy or progressive disease
No mutations
ATP Binding Site
Myristol Binding
Pocket
Asciminib
(n=39)
22 (56%)
M244V (n=3),
E355G, F359V,
T3151
ATP Binding Site
A337T (n=3),
P465
Mutations at baseline & end of treatment
F359C/V (n=3),
F317L (n=2), Y253H
Bosutinib
(n=30)
20 (67%)
T3151, V299L
None
M244V (n=2),
E255V, F317L,
Q252H
22View entire presentation