Compass Therapeutics Investor Presentation Deck
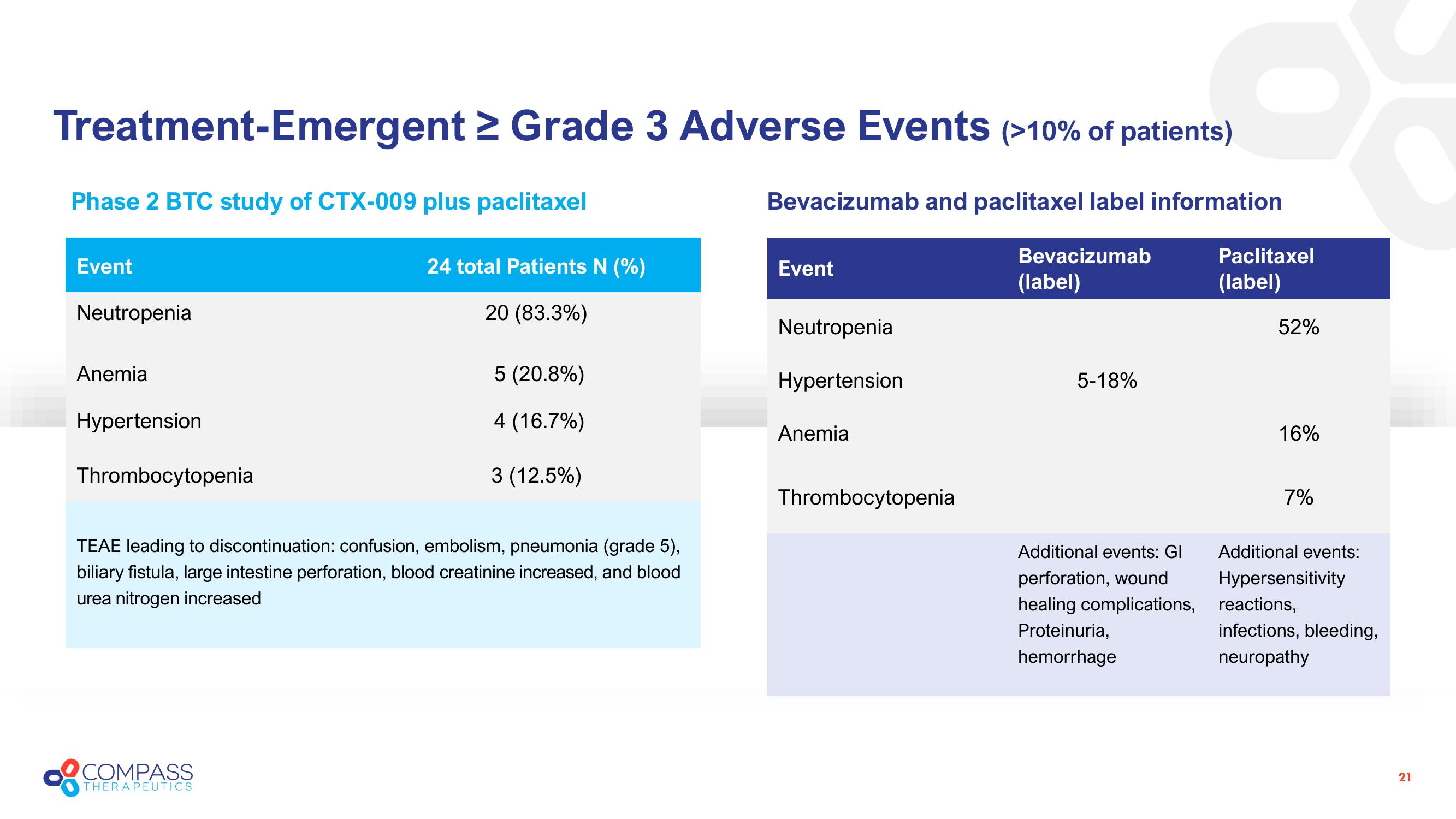
Treatment-Emergent ≥ Grade 3 Adverse Events (>10% of patients)
Phase 2 BTC study of CTX-009 plus paclitaxel
Bevacizumab and paclitaxel label information
Event
Neutropenia
Anemia
Hypertension
Thrombocytopenia
24 total Patients N (%)
20 (83.3%)
COMPASS
THERAPEUTICS
5 (20.8%)
4 (16.7%)
3 (12.5%)
TEAE leading to discontinuation: confusion, embolism, pneumonia (grade 5),
biliary fistula, large intestine perforation, blood creatinine increased, and blood
urea nitrogen increased
Event
Neutropenia
Hypertension
Anemia
Thrombocytopenia
Bevacizumab
(label)
5-18%
Additional events: Gl
perforation, wound
healing complications,
Proteinuria,
hemorrhage
Paclitaxel
(label)
52%
16%
7%
Additional events:
Hypersensitivity
reactions,
infections, bleeding,
neuropathy
21View entire presentation