Eleusis SPAC
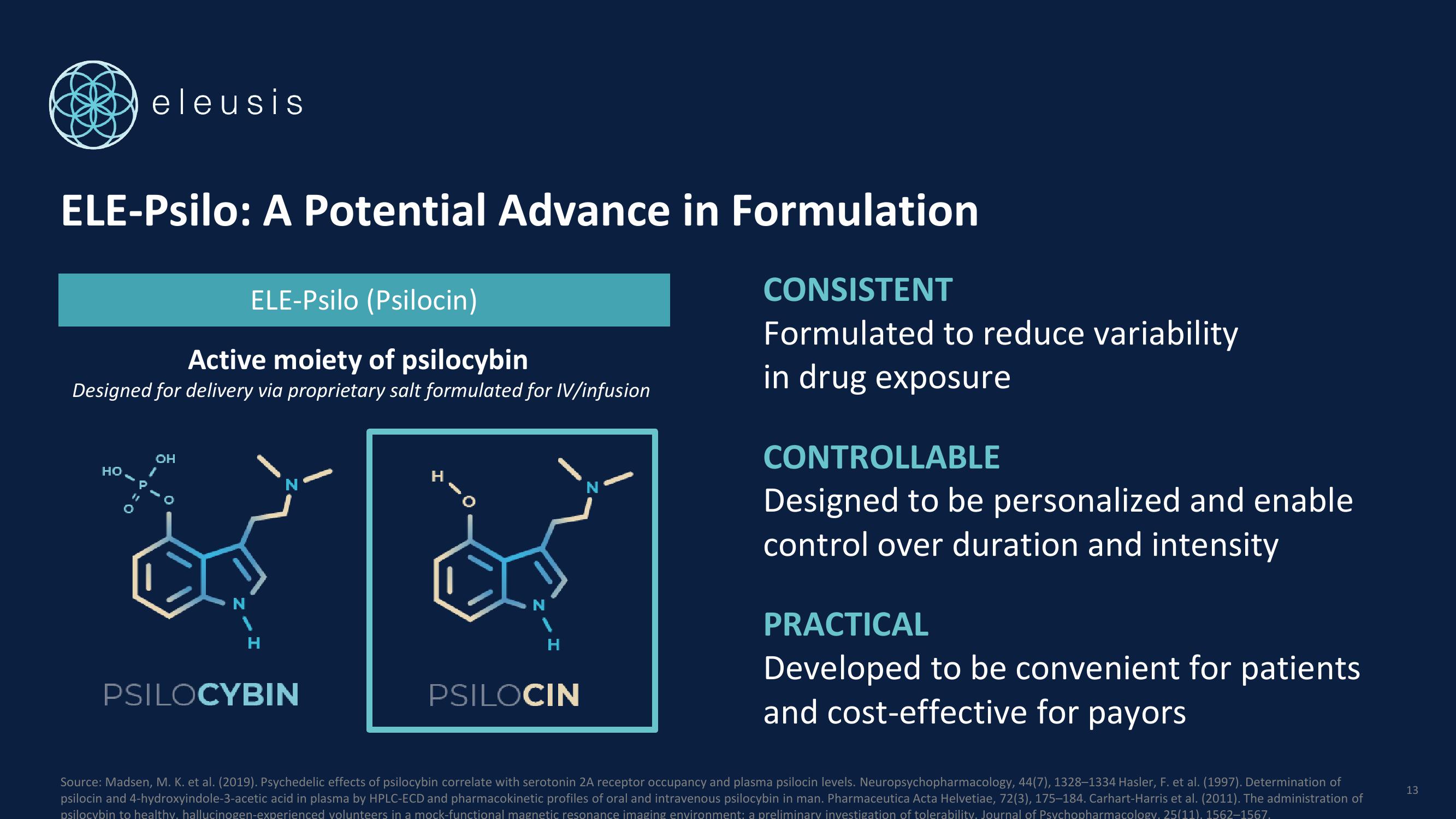
ELE-Psilo: A Potential Advance in Formulation
eleusis
ELE-Psilo (Psilocin)
Active moiety of psilocybin
Designed for delivery via proprietary salt formulated for IV/infusion
HO
O
OH
1
H
PSILOCYBIN
H
H
PSILOCIN
CONSISTENT
Formulated to reduce variability
in drug exposure
CONTROLLABLE
Designed to be personalized and enable
control over duration and intensity
PRACTICAL
Developed to be convenient for patients
and cost-effective for payors
Source: Madsen, M. K. et al. (2019). Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology, 44(7), 1328-1334 Hasler, F. et al. (1997). Determination of
psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharmaceutica Acta Helvetiae, 72(3), 175-184. Carhart-Harris et al. (2011). The administration of
psilocybin to healthy, hallucinogen-experienced volunteers in a mock-functional magnetic resonance imaging environment: a preliminary investigation of tolerability. Journal of Psychopharmacology, 25(11). 1562-1567.
13View entire presentation