Kymera Investor Day Presentation Deck
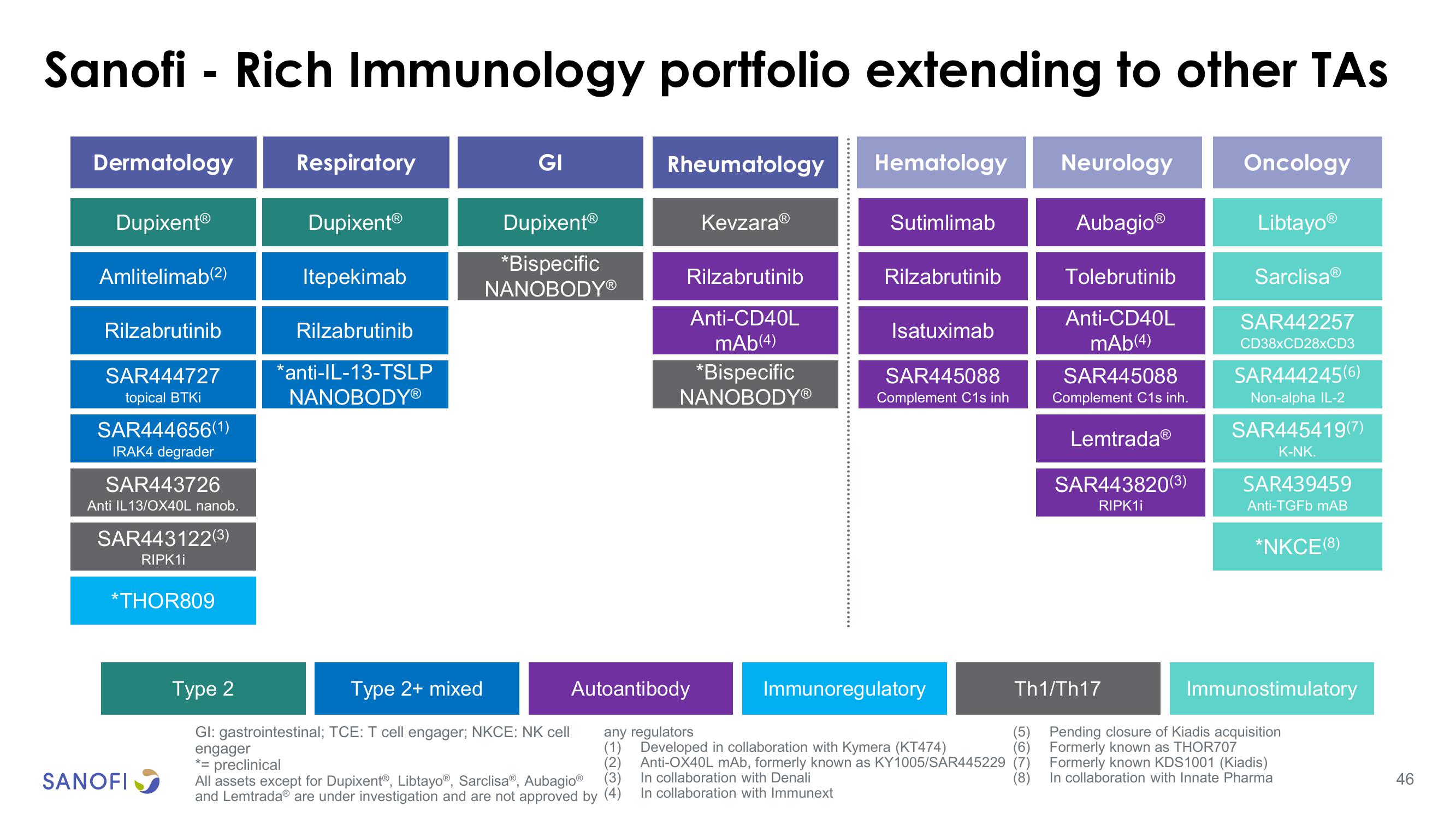
Sanofi - Rich Immunology portfolio extending to other TAs
Dermatology
DupixentⓇ
Amlitelimab(2)
Rilzabrutinib
SAR444727
topical BTKi
SAR444656(1)
IRAK4 degrader
SAR443726
Anti IL13/OX40L nanob.
SAR443122(3)
RIPK1i
*THOR809
SANOFI
Respiratory
DupixentⓇ
Itepekimab
Rilzabrutinib
*anti-IL-13-TSLP
NANOBODYⓇ
GI
DupixentⓇ
*Bispecific
NANOBODYⓇ
Type 2
Type 2+ mixed
Gl: gastrointestinal; TCE: T cell engager; NKCE: NK cell
engager
Rheumatology
*= preclinical
All assets except for Dupixent®, Libtayo®, Sarclisa®, AubagioⓇ (3)
and LemtradaⓇ are under investigation and are not approved by (4)
Rilzabrutinib
Anti-CD40L
mAb(4)
Autoantibody
KevzaraⓇ
*Bispecific
NANOBODYⓇ
any regulators
(1)
(2)
Hematology Neurology
Sutimlimab
Rilzabrutinib
Isatuximab
SAR445088
Complement C1s inh
Immunoregulatory
(5)
Developed in collaboration with Kymera (KT474)
(6)
Anti-OX40L mAb, formerly known as KY1005/SAR445229 (7)
In collaboration with Denali
(8)
In collaboration with Immunext
AubagioⓇ
Tolebrutinib
Anti-CD40L
mAb(4)
SAR445088
Complement C1s inh.
LemtradaⓇ
SAR443820(3)
RIPK1i
Th1/Th17
Oncology
LibtayoⓇ
SarclisaⓇ
SAR442257
CD38xCD28xCD3
SAR444245(6)
Non-alpha IL-2
SAR445419(7)
K-NK.
SAR439459
Anti-TGFb mAB
*NKCE(8)
Immunostimulatory
Pending closure of Kiadis acquisition
Formerly known as THOR707
Formerly known KDS1001 (Kiadis)
In collaboration with Innate Pharma
46View entire presentation