BioNTech Investor Day Presentation Deck
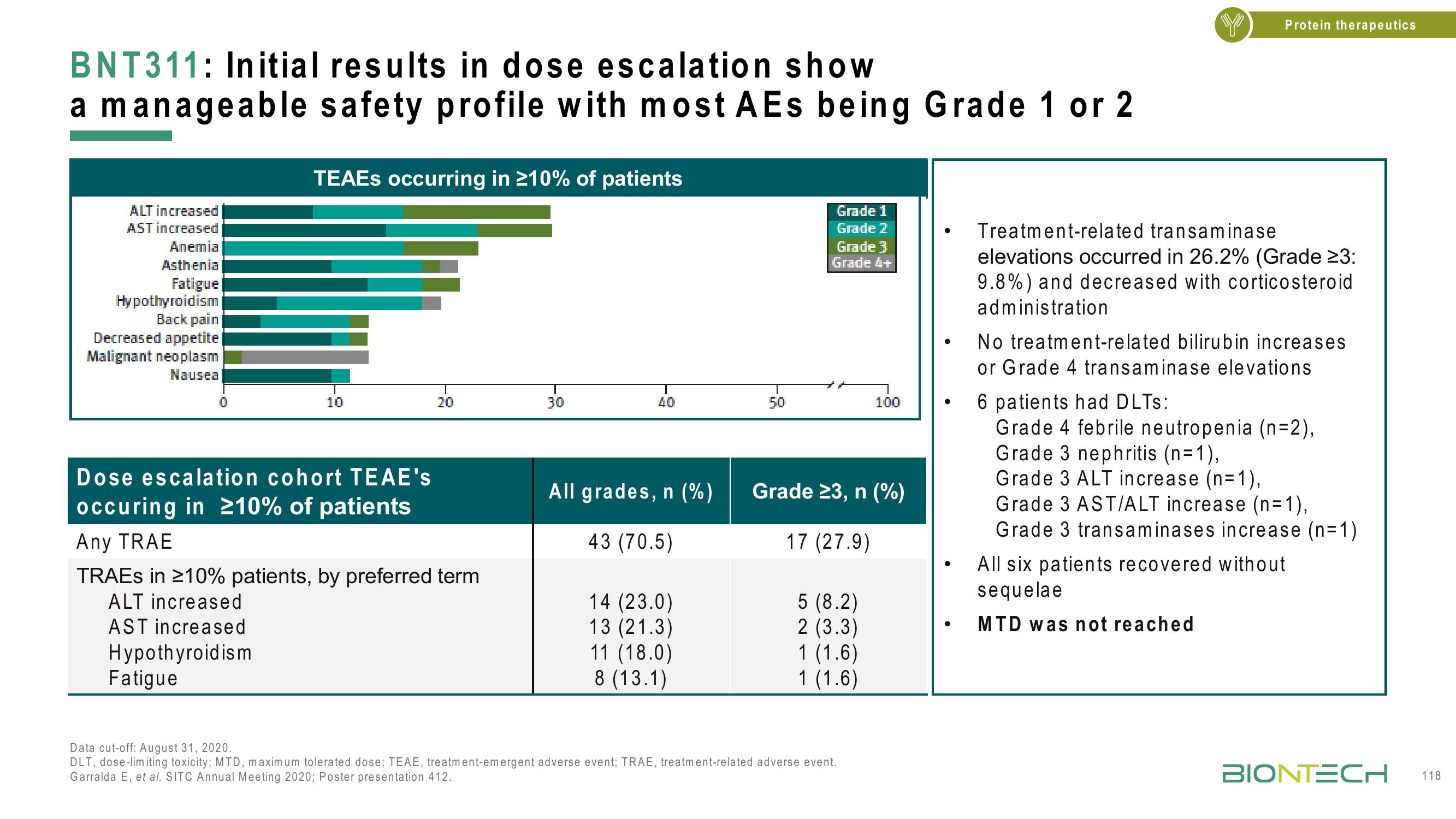
BNT311: Initial results in dose escalation show
a manageable safety profile with most AEs being Grade 1 or 2
ALT increased
AST increased
Anemia
Asthenia
Fatigue
Hypothyroidism
Back pain
Decreased appetite
Malignant neoplasm
Nausea
TEAES occurring in ≥10% of patients
10
20
Dose escalation cohort TEAE's
occuring in ≥10% of patients
Any TRAE
TRAES in ≥10% patients, by preferred term
ALT increased
AST increased
Hypothyroidism
Fatigue
30
T
40
All grades, n (%)
43 (70.5)
14 (23.0)
13 (21.3)
11 (18.0)
8 (13.1)
50
Grade 1
Grade 2
Grade 3
Grade 4+
Grade 23, n (%)
17 (27.9)
5 (8.2)
2 (3.3)
1 (1.6)
1 (1.6)
100
Data cut-off: August 31, 2020.
DLT, dose-limiting toxicity; MTD, maximum tolerated dose; TEAE, treatment-emergent adverse event; TRAE, treatment-related adverse event.
Garralda E, et al. SITC Annual Meeting 2020; Poster presentation 412.
Protein therapeutics
Treatment-related transaminase
elevations occurred in 26.2% (Grade 23:
9.8%) and decreased with corticosteroid
administration
No treatment-related bilirubin increases
or Grade 4 transaminase elevations
6 patients had DLTs:
Grade 4 febrile neutropenia (n=2),
Grade 3 nephritis (n=1),
Grade 3 ALT increase (n=1),
Grade 3 AST/ALT increase (n=1),
Grade 3 transaminases increase (n=1)
All six patients recovered without
sequelae
MTD was not reached
BIONTECH
118View entire presentation