Kymera Investor Presentation Deck
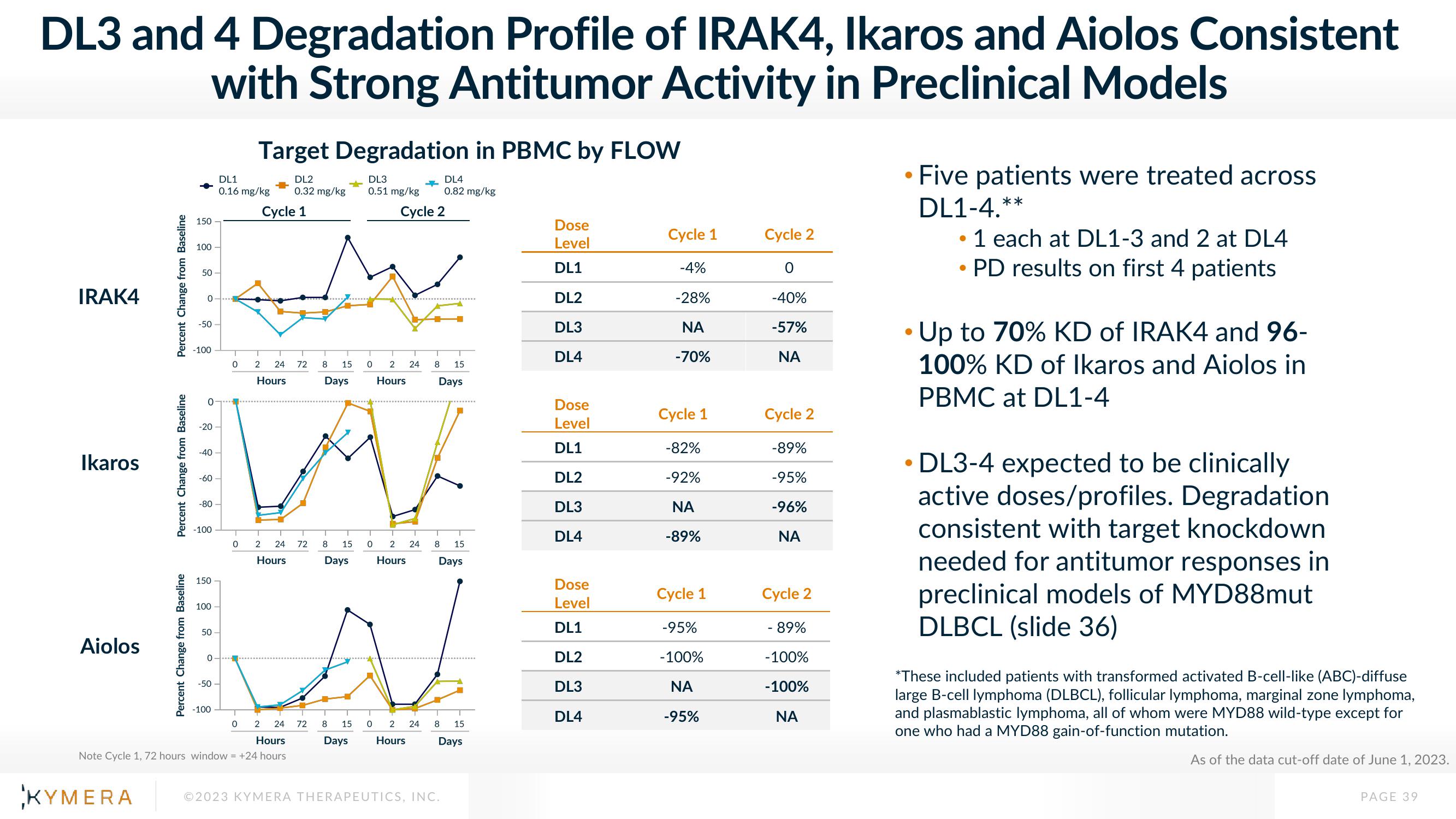
DL3 and 4 Degradation Profile of IRAK4, Ikaros and Aiolos Consistent
with Strong Antitumor Activity in Preclinical Models
IRAK4
Ikaros
Aiolos
Percent Change from Baseline
Percent Change from Baseline
150
100
50
0
-50
-100
0
-20-
-40
-60
-80
-100
150
100
50
-50
Target Degradation in PBMC by FLOW
DL1
DL2
DL3
DL4
0.16 mg/kg 0.32 mg/kg 0.51 mg/kg 0.82 mg/kg
Cycle 1
Cycle 2
M
-100
T
0
0
T
2 24
Hours
T
2
24
Hours
02 24
Hours
Note Cycle 1, 72 hours window = +24 hours
72
72
72
T
8
Days
8
15 0
+
15
Days
T T
2
Hours
-
T
24 8
T
15
Days
T T
0 2 24 8
Hours
15
Days
8 15 0 2 24 8 15
Days Hours Days
KYMERA Ⓒ2023 KYMERA THERAPEUTICS, INC.
Dose
Level
Cycle 1
DL1
-4%
DL2
-28%
DL3
ΝΑ
DL4
-70%
H
Dose
Cycle 1
Level
DL1
-82%
D
-92%
DL3
ΝΑ
-89%
DL4
Dose
Level
DL1
DL2
DL3
DL4
Cycle 1
-95%
-100%
ΝΑ
-95%
Cycle 2
0
-40%
-57%
ΝΑ
Cycle 2
-89%
-95%
-96%
ΝΑ
Cycle 2
- 89%
-100%
-100%
ΝΑ
Five patients were treated across
DL1-4.**
●
1 each at DL1-3 and 2 at DL4
• PD results on first 4 patients
Up to 70% KD of IRAK4 and 96-
100% KD of Ikaros and Aiolos in
PBMC at DL1-4
DL3-4 expected to be clinically
active doses/profiles. Degradation
consistent with target knockdown
needed for antitumor responses in
preclinical models of MYD88mut
DLBCL (slide 36)
*These included patients with transformed activated B-cell-like (ABC)-diffuse
large B-cell lymphoma (DLBCL), follicular lymphoma, marginal zone lymphoma,
and plasmablastic lymphoma, all of whom were MYD88 wild-type except for
one who had a MYD88 gain-of-function mutation.
As of the data cut-off date of June 1, 2023.
PAGE 39View entire presentation