Ocuphire Pharma Investor Day Presentation Deck
P
83
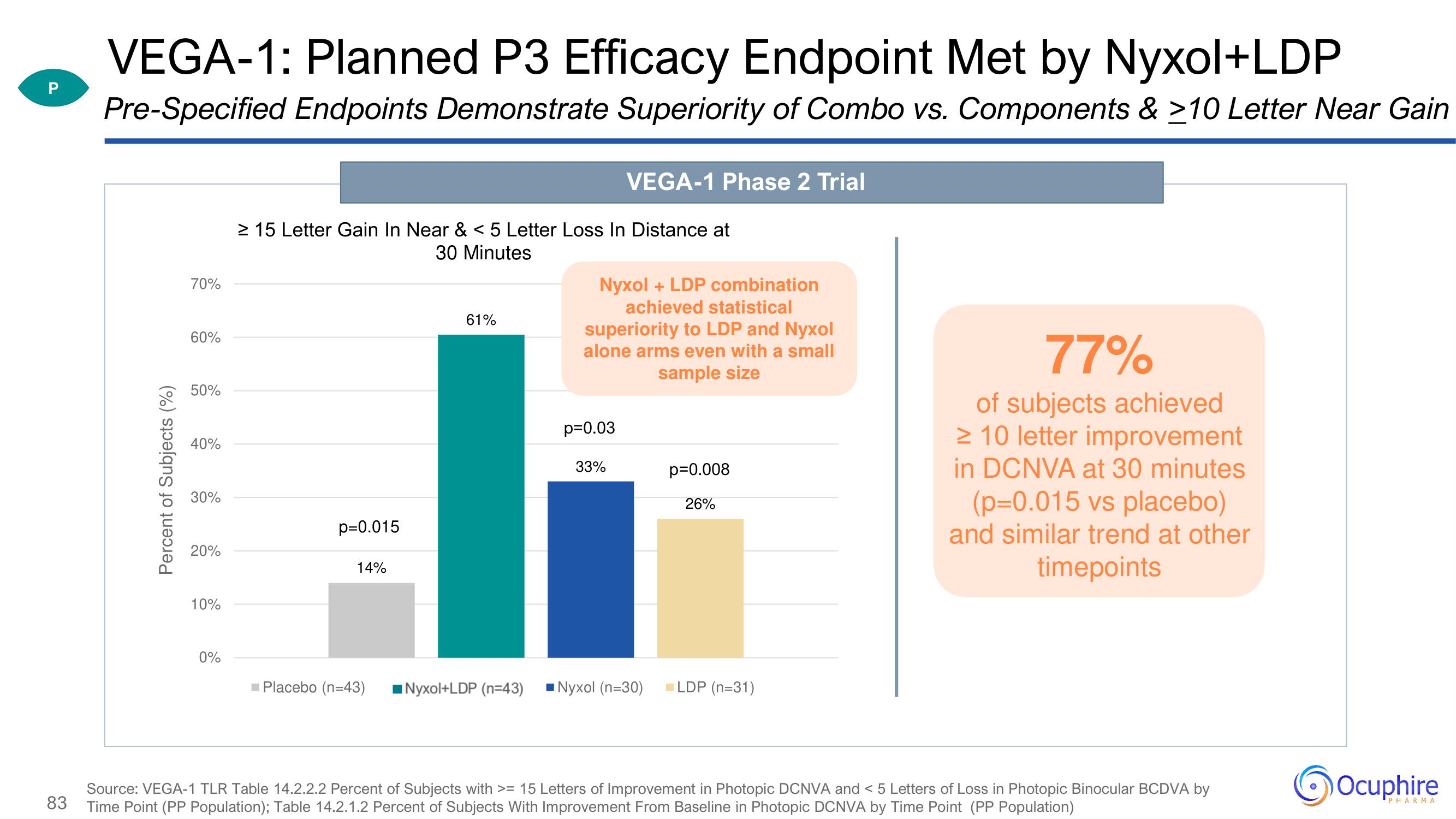
VEGA-1: Planned P3 Efficacy Endpoint Met by Nyxol+LDP
Pre-Specified Endpoints Demonstrate Superiority of Combo vs. Components & ≥10 Letter Near Gain
Percent of Subjects (%)
70%
60%
50%
40%
30%
20%
10%
0%
≥ 15 Letter Gain In Near & < 5 Letter Loss In Distance at
30 Minutes
p=0.015
14%
61%
VEGA-1 Phase 2 Trial
Nyxol + LDP combination
achieved statistical
superiority to LDP and Nyxol
alone arms even with a small
sample size
p=0.03
33%
Placebo (n=43) Nyxol+LDP (n=43) Nyxol (n=30)
p=0.008
26%
LDP (n=31)
77%
of subjects achieved
> 10 letter improvement
in DCNVA at 30 minutes
(p=0.015 vs placebo)
and similar trend at other
timepoints
Source: VEGA-1 TLR Table 14.2.2.2 Percent of Subjects with >= 15 Letters of Improvement in Photopic DCNVA and < 5 Letters of Loss in Photopic Binocular BCDVA by
Time Point (PP Population); Table 14.2.1.2 Percent of Subjects With Improvement From Baseline in Photopic DCNVA by Time Point (PP Population)
Ocuphire
PHARMAView entire presentation