Connecting Innovation to Purpose
Safety and Dose Modifications
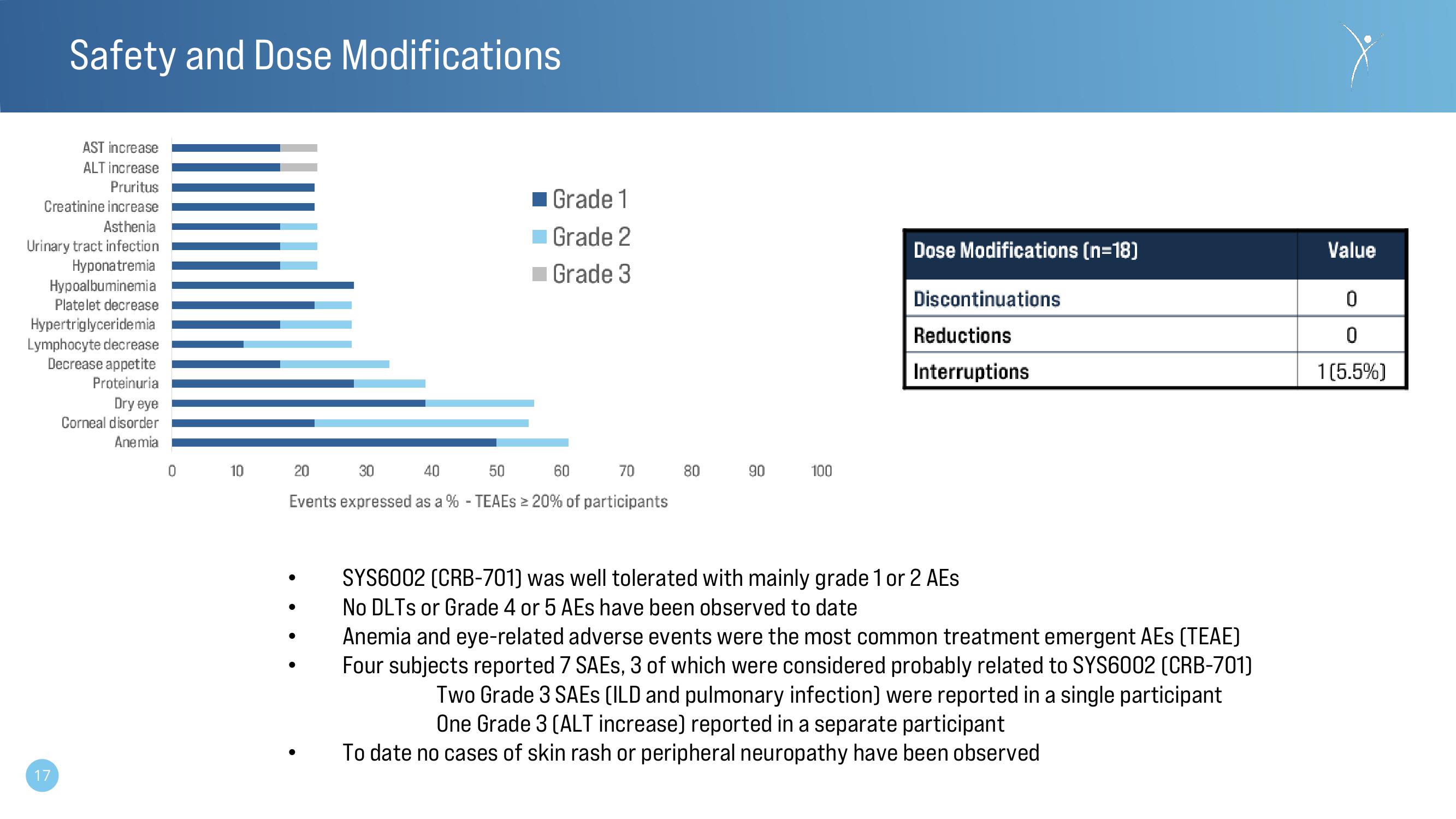
AST increase
ALT increase
Pruritus
Creatinine increase
Asthenia
Urinary tract infection
Hyponatremia
Hypoalbuminemia
Platelet decrease
Hypertriglyceridemia
Lymphocyte decrease
Decrease appetite
Proteinuria
17
Dry eye
Corneal disorder
Anemia
0
10
Grade 1
Grade 2
Grade 3
20
30
40
50
60 70 80
Events expressed as a % - TEAEs ≥ 20% of participants
90
100
Dose Modifications (n=18)
Discontinuations
Reductions
Interruptions
SYS6002 (CRB-701) was well tolerated with mainly grade 1 or 2 AEs
No DLTS or Grade 4 or 5 AEs have been observed to date
Anemia and eye-related adverse events were the most common treatment emergent AEs [TEAE)
Four subjects reported 7 SAES, 3 of which were considered probably related to SYS6002 (CRB-701)
Two Grade 3 SAES (ILD and pulmonary infection) were reported in a single participant
One Grade 3 (ALT increase) reported in a separate participant
To date no cases of skin rash or peripheral neuropathy have been observed
Value
0
0
1 [5.5%)View entire presentation