AstraZeneca Results Presentation Deck
Appendix: Rare Diseases - 'What's next'
Pipeline progress continued
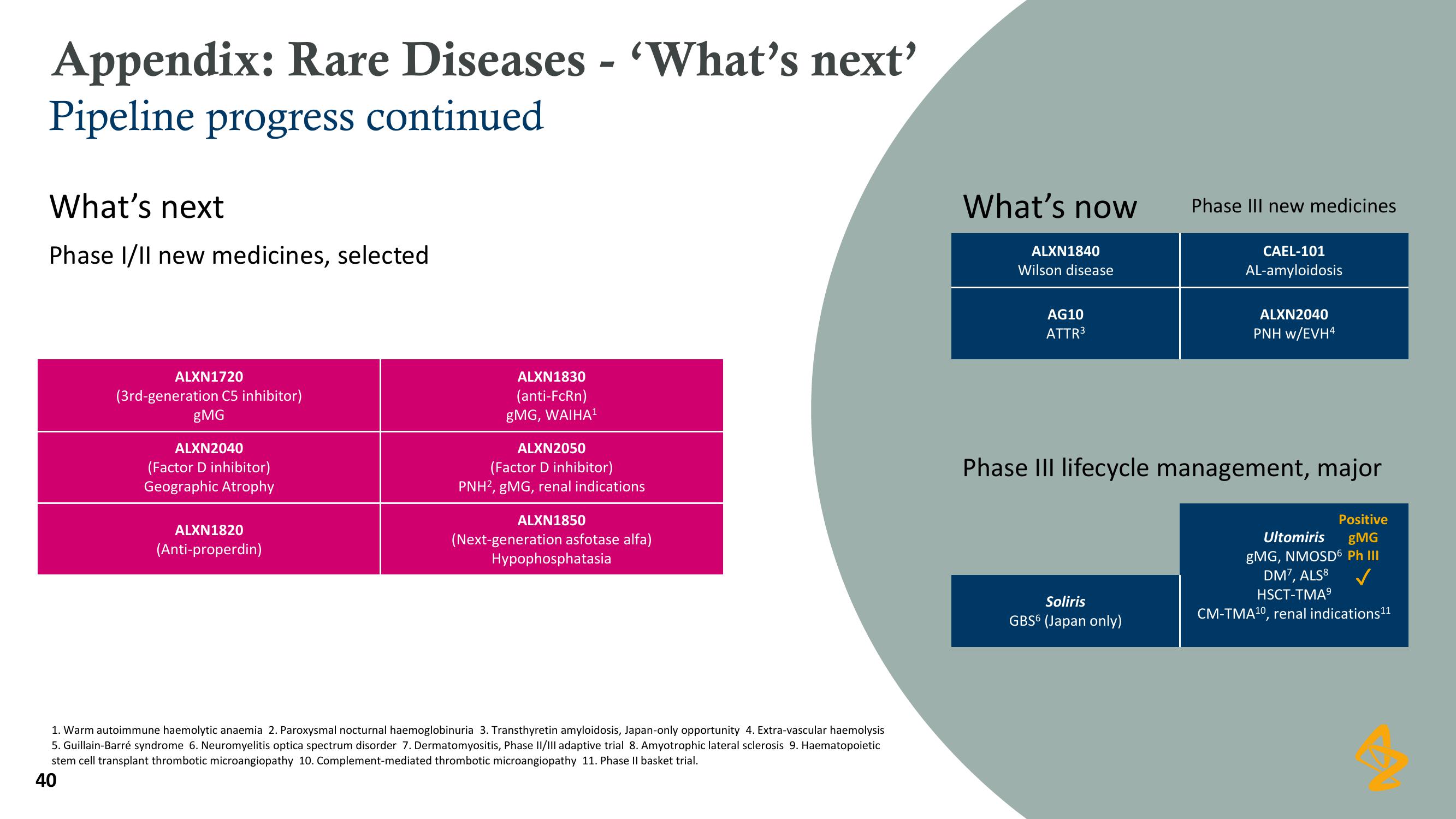
What's next
Phase I/II new medicines, selected
ALXN1720
(3rd-generation C5 inhibitor)
gMG
ALXN2040
(Factor D inhibitor)
Geographic Atrophy
ALXN1820
(Anti-properdin)
ALXN1830
(anti-FcRn)
gMG, WAIHA¹
ALXN2050
(Factor D inhibitor)
PNH2, gMG, renal indications
ALXN1850
(Next-generation asfotase alfa)
Hypophosphatasia
1. Warm autoimmune haemolytic anaemia 2. Paroxysmal nocturnal haemoglobinuria 3. Transthyretin amyloidosis, Japan-only opportunity 4. Extra-vascular haemolysis
5. Guillain-Barré syndrome 6. Neuromyelitis optica spectrum disorder 7. Dermatomyositis, Phase II/III adaptive trial 8. Amyotrophic lateral sclerosis 9. Haematopoietic
stem cell transplant thrombotic microangiopathy 10. Complement-mediated thrombotic microangiopathy 11. Phase II basket trial.
40
What's now
ALXN1840
Wilson disease
AG10
ATTR³
Phase III new medicines
Soliris
GBS6 (Japan only)
CAEL-101
AL-amyloidosis
ALXN2040
PNH w/EVH4
Phase III lifecycle management, major
Positive
gMG
gMG, NMOSD6 Ph III
Ultomiris
DM7, ALS8
HSCT-TMA⁹
CM-TMA¹0, renal indications1¹¹View entire presentation