BioAtla Investor Conference Presentation Deck
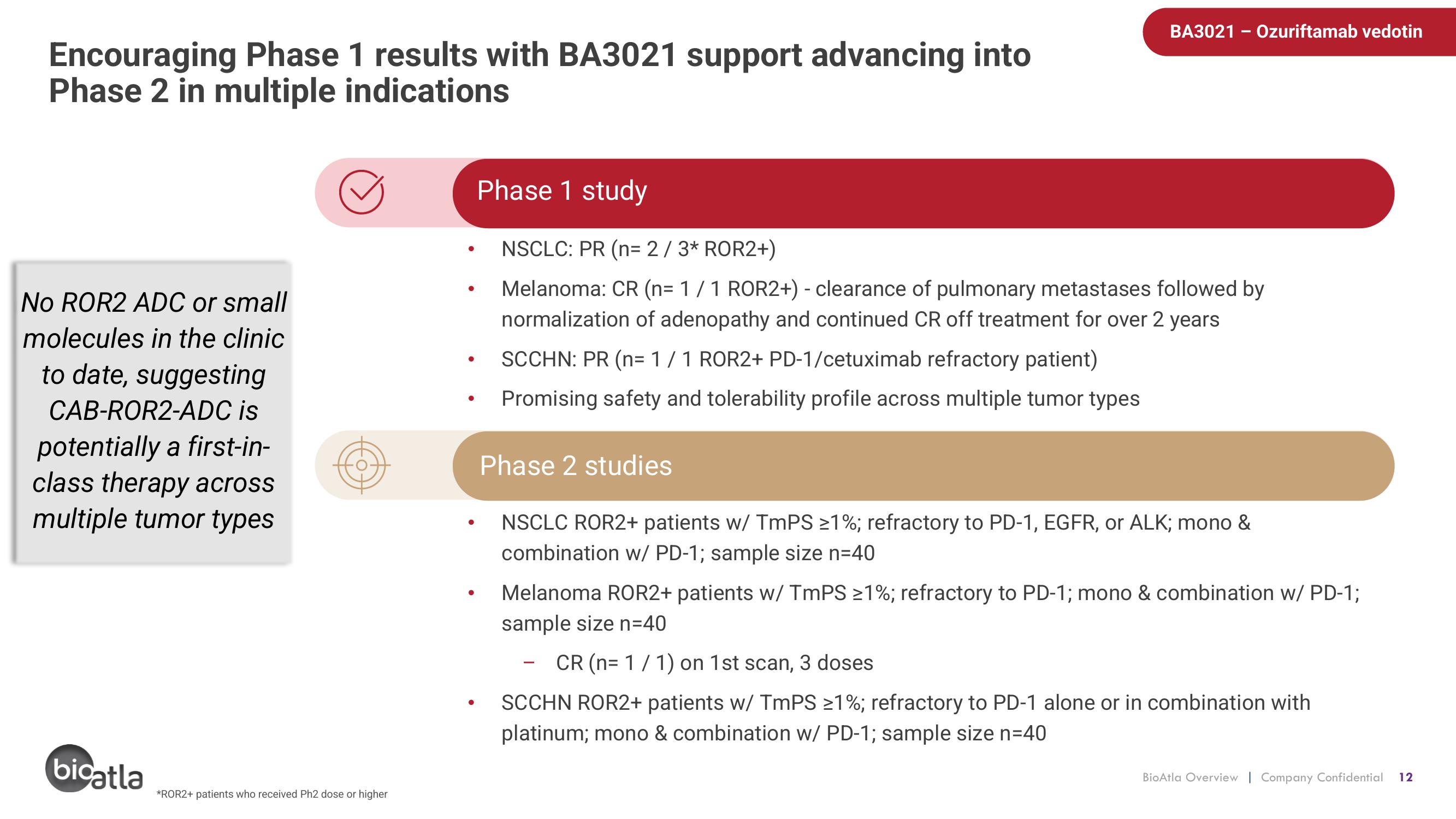
Encouraging Phase 1 results with BA3021 support advancing into
Phase 2 in multiple indications
No ROR2 ADC or small
molecules in the clinic
to date, suggesting
CAB-ROR2-ADC is
potentially a first-in-
class therapy across
multiple tumor types
bicatla
*ROR2+ patients who received Ph2 dose or higher
●
●
BA3021 - Ozuriftamab vedotin
Phase 1 study
NSCLC: PR (n= 2/ 3* ROR2+)
Melanoma: CR (n= 1 / 1 ROR2+) - clearance of pulmonary metastases followed by
normalization of adenopathy and continued CR off treatment for over 2 years
SCCHN: PR (n= 1 / 1 ROR2+ PD-1/cetuximab refractory patient)
Promising safety and tolerability profile across multiple tumor types
Phase 2 studies
NSCLC ROR2+ patients w/ TmPS ≥1%; refractory to PD-1, EGFR, or ALK; mono &
combination w/ PD-1; sample size n=40
Melanoma ROR2+ patients w/ TmPS ≥1%; refractory to PD-1; mono & combination w/ PD-1;
sample size n=40
CR (n=1 / 1) on 1st scan, 3 doses
SC OR2+ patients w/ TmPS ≥1%; refractory to PD-1 alone or in combination with
platinum; mono & combination w/ PD-1; sample size n=40
BioAtla Overview | Company Confidential 12View entire presentation