BioAtla Investor Presentation Deck
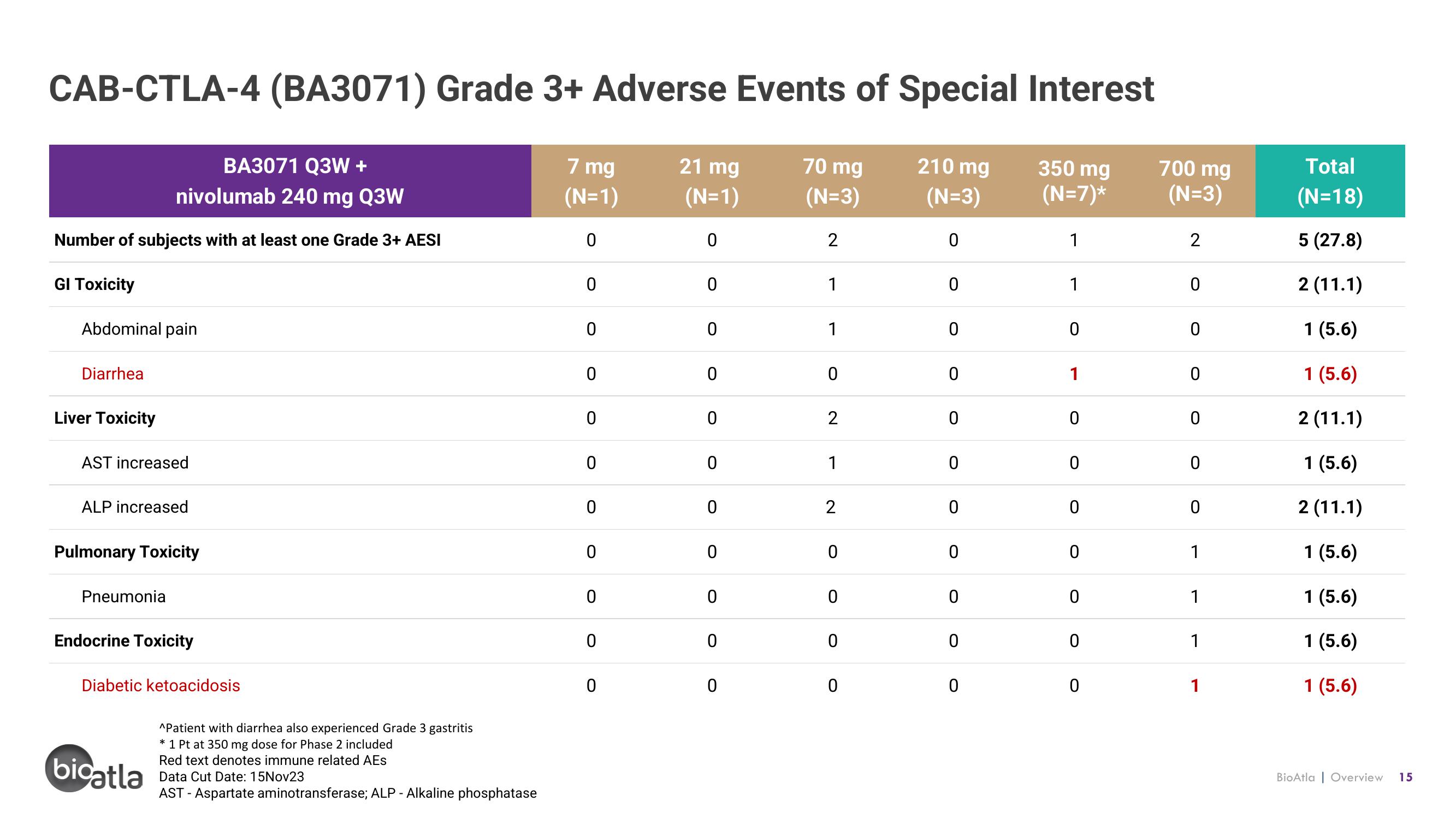
CAB-CTLA-4 (BA3071) Grade 3+ Adverse Events of Special Interest
7 mg
(N=1)
0
BA3071 Q3W +
nivolumab 240 mg Q3W
Number of subjects with at least one Grade 3+ AESI
GI Toxicity
Abdominal pain
Diarrhea
Liver Toxicity
AST increased
ALP increased
Pulmonary Toxicity
Pneumonia
Endocrine Toxicity
Diabetic ketoacidosis
bicatla
^Patient with diarrhea also experienced Grade 3 gastritis
* 1 Pt at 350 mg dose for Phase 2 included
Red text denotes immune related AES
Data Cut Date: 15Nov23
AST - Aspartate aminotransferase; ALP - Alkaline phosphatase
0
0
0
0
0
0
0
0
0
0
21 mg
(N=1)
0
0
0
0
0
0
0
0
0
0
0
70 mg
(N=3)
2
1
1
0
2
1
2
0
0
0
0
210 mg
(N=3)
0
0
0
0
0
0
0
0
0
0
0
350 mg
(N=7)*
1
1
0
1
0
0
0
0
0
0
0
700 mg
(N=3)
2
0
0
0
0
0
0
1
1
1
1
Total
(N=18)
5 (27.8)
2 (11.1)
1 (5.6)
1 (5.6)
2 (11.1)
1 (5.6)
2 (11.1)
1 (5.6)
1 (5.6)
1 (5.6)
1 (5.6)
BioAtla| Overview
15View entire presentation