Vaxcyte Corporate Presentation
24
24
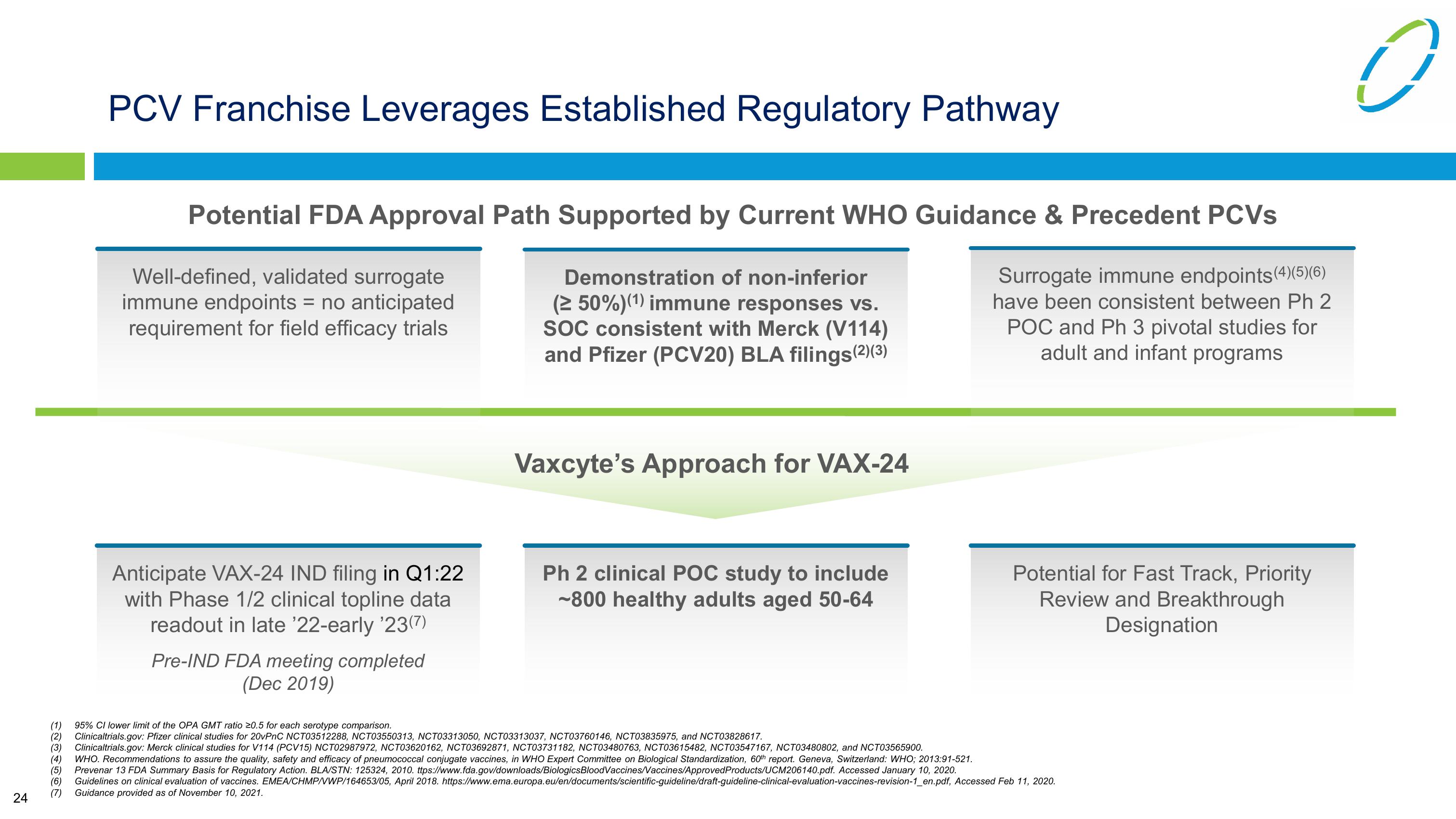
PCV Franchise Leverages Established Regulatory Pathway
о
Potential FDA Approval Path Supported by Current WHO Guidance & Precedent PCVs
Well-defined, validated surrogate
immune endpoints = no anticipated
requirement for field efficacy trials
Demonstration of non-inferior
(≥50%)(1) immune responses vs.
SOC consistent with Merck (V114)
and Pfizer (PCV20) BLA filings (2)(3)
Surrogate immune endpoints (4)(5)(6)
have been consistent between Ph 2
POC and Ph 3 pivotal studies for
adult and infant programs
Anticipate VAX-24 IND filing in Q1:22
with Phase 1/2 clinical topline data
readout in late '22-early '23(7)
Pre-IND FDA meeting completed
(Dec 2019)
Vaxcyte's Approach for VAX-24
Ph 2 clinical POC study to include
~800 healthy adults aged 50-64
Potential for Fast Track, Priority
Review and Breakthrough
Designation
(1)
(2)
(3)
(4)
(5)
95% CI lower limit of the OPA GMT ratio ≥0.5 for each serotype comparison.
Clinicaltrials.gov: Pfizer clinical studies for 20vPnC NCT03512288, NCT03550313, NCT03313050, NCT03313037, NCT03760146, NCT03835975, and NCT03828617.
Clinicaltrials.gov: Merck clinical studies for V114 (PCV15) NCT02987972, NCT03620162, NCT03692871, NCT03731182, NCT03480763, NCT03615482, NCT03547167, NCT03480802, and NCT03565900.
WHO. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines, in WHO Expert Committee on Biological Standardization, 60th report. Geneva, Switzerland: WHO; 2013:91-521.
Prevenar 13 FDA Summary Basis for Regulatory Action. BLA/STN: 125324, 2010. ttps://www.fda.gov/downloads/Biologics Blood Vaccines/Vaccines/Approved Products/UCM206140.pdf. Accessed January 10, 2020.
(6) Guidelines on clinical evaluation of vaccines. EMEA/CHMP/VWP/164653/05, April 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-clinical-evaluation-vaccines-revision-1_en.pdf, Accessed Feb 11, 2020.
(7) Guidance provided as of November 10, 2021.View entire presentation