Dare Bioscience Investor Presentation Deck
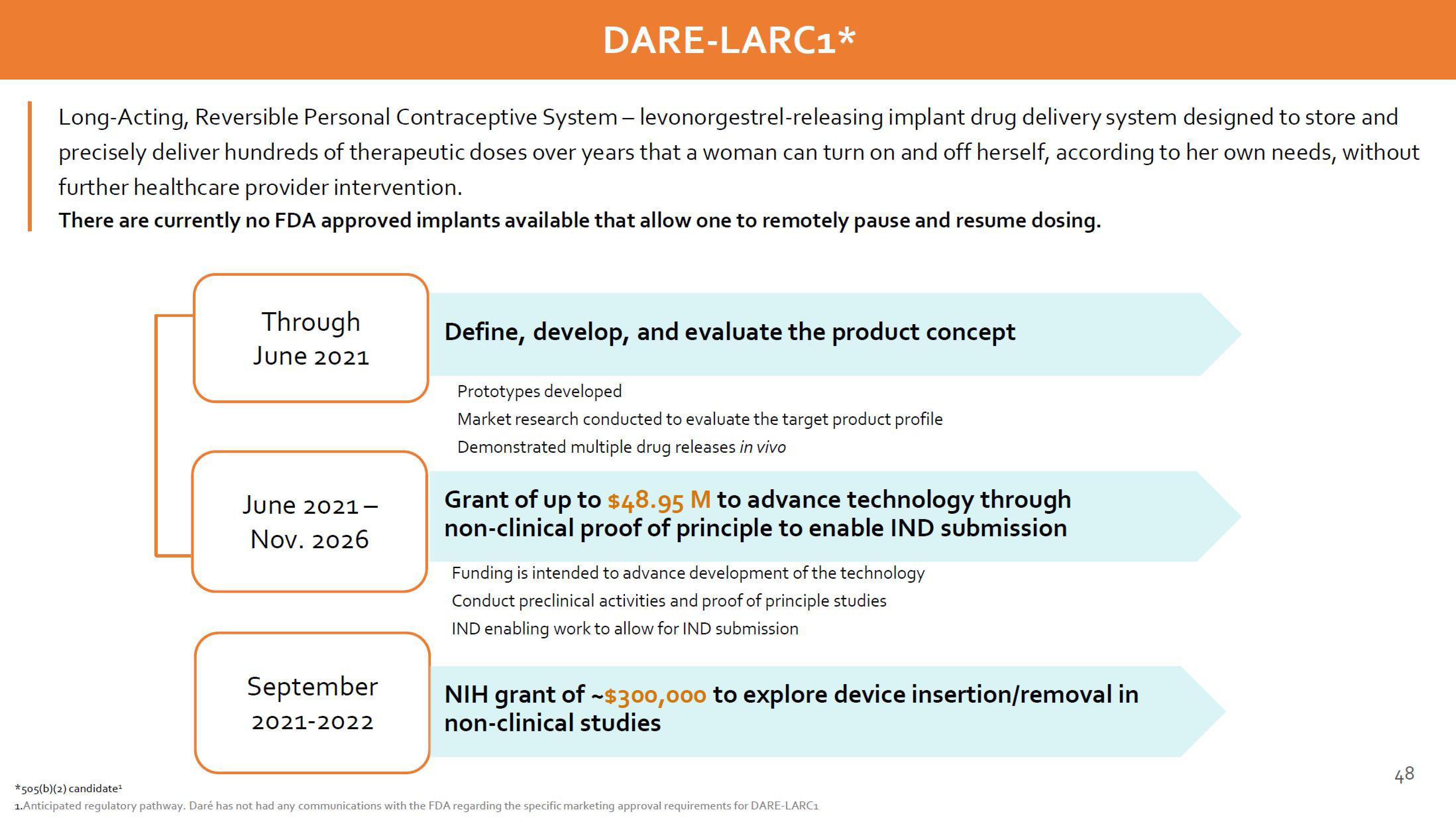
Long-Acting, Reversible Personal Contraceptive System - levonorgestrel-releasing implant drug delivery system designed to store and
precisely deliver hundreds of therapeutic doses over years that a woman can turn on and off herself, according to her own needs, without
further healthcare provider intervention.
There are currently no FDA approved implants available that allow one to remotely pause and resume dosing.
Through
June 2021
June 2021-
Nov. 2026
DARE-LARC1*
September
2021-2022
Define, develop, and evaluate the product concept
Prototypes developed
Market research conducted to evaluate the target product profile
Demonstrated multiple drug releases in vivo
Grant of up to $48.95 M to advance technology through
non-clinical proof of principle to enable IND submission
Funding is intended to advance development of the technology
Conduct preclinical activities and proof of principle studies
IND enabling work to allow for IND submission
NIH grant of ~$300,000 to explore device insertion/removal in
non-clinical studies
*505(b)(2) candidate¹
1.Anticipated regulatory pathway. Daré has not had any communications with the FDA regarding the specific marketing approval requirements for DARE-LARC1
48View entire presentation