BenevolentAI Investor Conference Presentation Deck
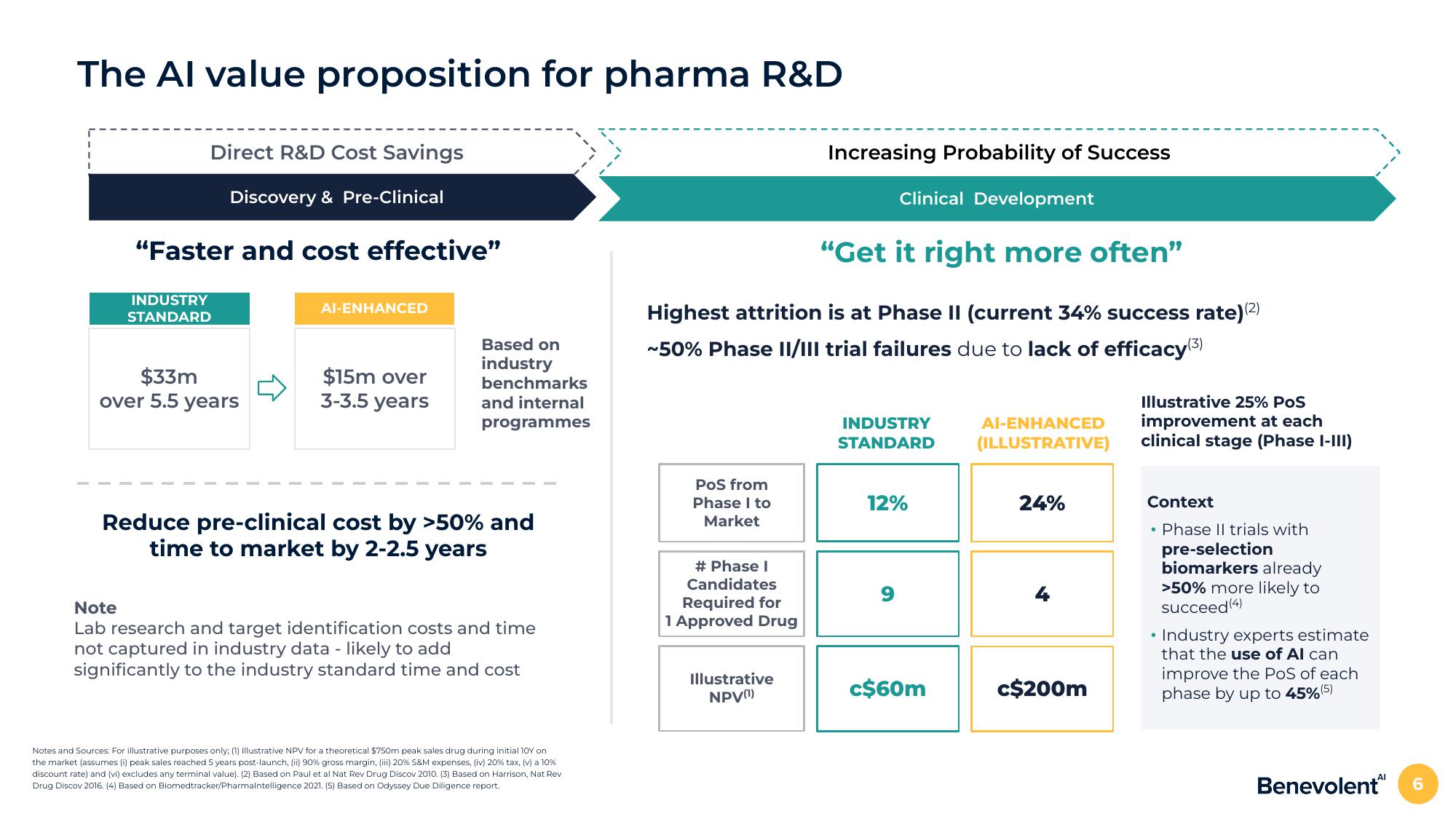
The Al value proposition for pharma R&D
Direct R&D Cost Savings
Discovery & Pre-Clinical
"Faster and cost effective"
INDUSTRY
STANDARD
$33m
over 5.5 years
AI-ENHANCED
$15m over
3-3.5 years
Based on
industry
benchmarks
and internal
programmes
Reduce pre-clinical cost by >50% and
time to market by 2-2.5 years
Note
Lab research and target identification costs and time
not captured in industry data - likely to add
significantly to the industry standard time and cost
Notes and Sources: For illustrative purposes only; (1) Illustrative NPV for a theoretical $750m peak sales drug during initial 10Y on
the market (assumes (i) peak sales reached 5 years post-launch, (ii) 90% gross margin, (iii) 20% S&M expenses, (iv) 20% tax, (v) a 10%
discount rate) and (vi) excludes any terminal value). (2) Based on Paul et al Nat Rev Drug Discov 2010. (3) Based on Harrison, Nat Rev
Drug Discov 2016. (4) Based on Biomedtracker/Pharmalntelligence 2021. (5) Based on Odyssey Due Diligence report.
PoS from
Phase I to
Market
"Get it right more often"
Highest attrition is at Phase II (current 34% success rate) (2)
~50% Phase II/III trial failures due to lack of efficacy (3)
# Phase I
Candidates
Required for
1 Approved Drug
Increasing Probability of Success
Illustrative
NPV(¹)
Clinical Development
INDUSTRY
STANDARD
12%
9
c$60m
AI-ENHANCED
(ILLUSTRATIVE)
24%
c$200m
Illustrative 25% POS
improvement at each
clinical stage (Phase I-III)
Context
• Phase II trials with
pre-selection
biomarkers already
>50% more likely to
succeed (4)
Industry experts estimate
that the use of Al can
improve the PoS of each
phase by up to 45% (5)
Benevolent
6View entire presentation