Calliditas Therapeutics IPO Presentation Deck
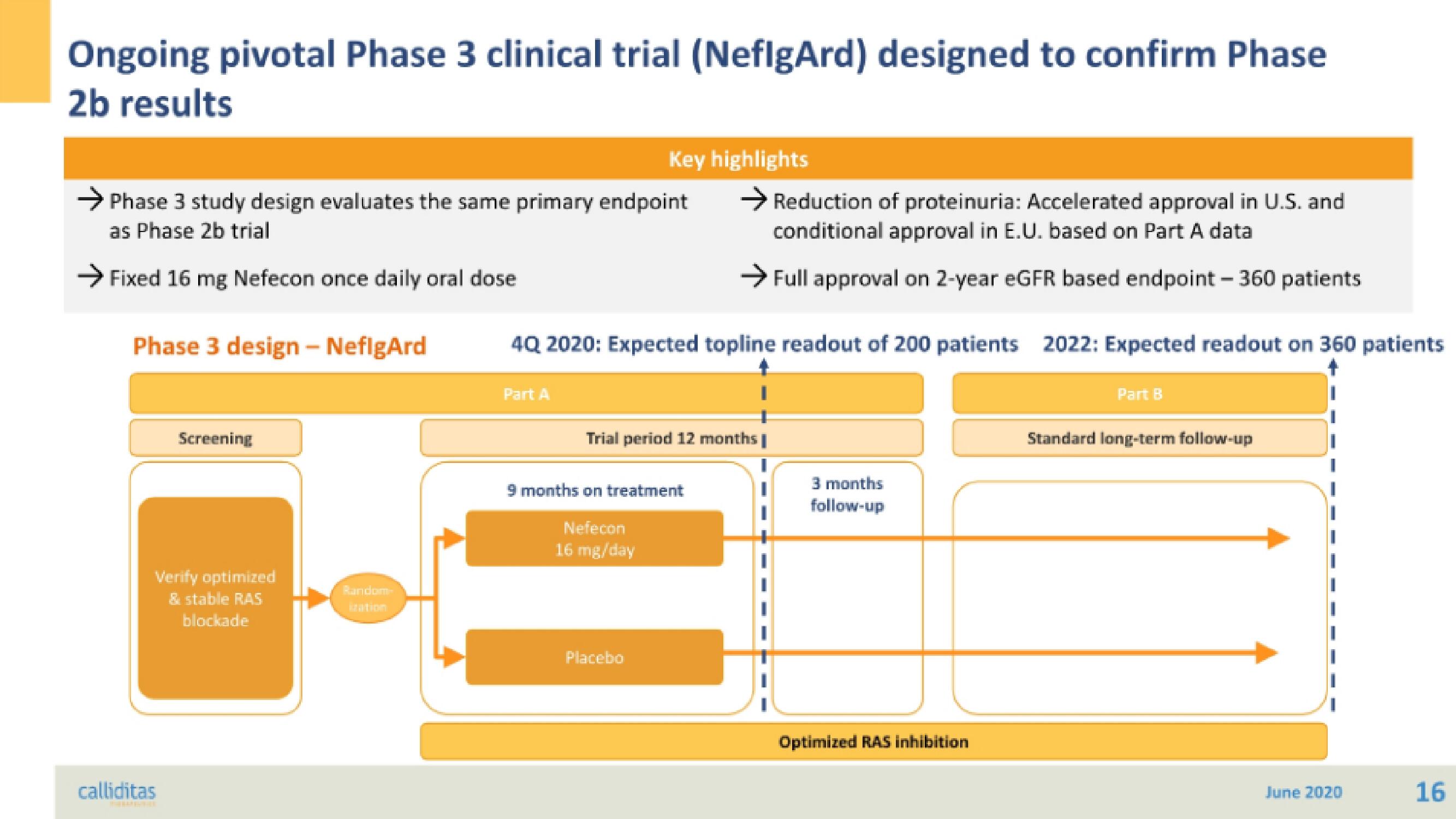
Ongoing pivotal Phase 3 clinical trial (NeflgArd) designed to confirm Phase
2b results
→ Phase 3 study design evaluates the same primary endpoint
as Phase 2b trial
→ Fixed 16 mg Nefecon once daily oral dose
Phase 3 design - NeflgArd
Screening
Verify optimized
& stable RAS
blockade
calliditas
Bandom-
Key highlights
Part A
4Q 2020: Expected topline readout of 200 patients 2022: Expected readout on 360 patients
→ Reduction of proteinuria: Accelerated approval in U.S. and
conditional approval in E.U. based on Part A data
→Full approval on 2-year eGFR based endpoint - 360 patients
Trial period 12 months |
9 months on treatment
Nefecon
16 mg/day
Placebo
3 months
follow-up
Optimized RAS inhibition
Part B
Standard long-term follow-up
June 2020
16View entire presentation