BioNTech Investor Day Presentation Deck
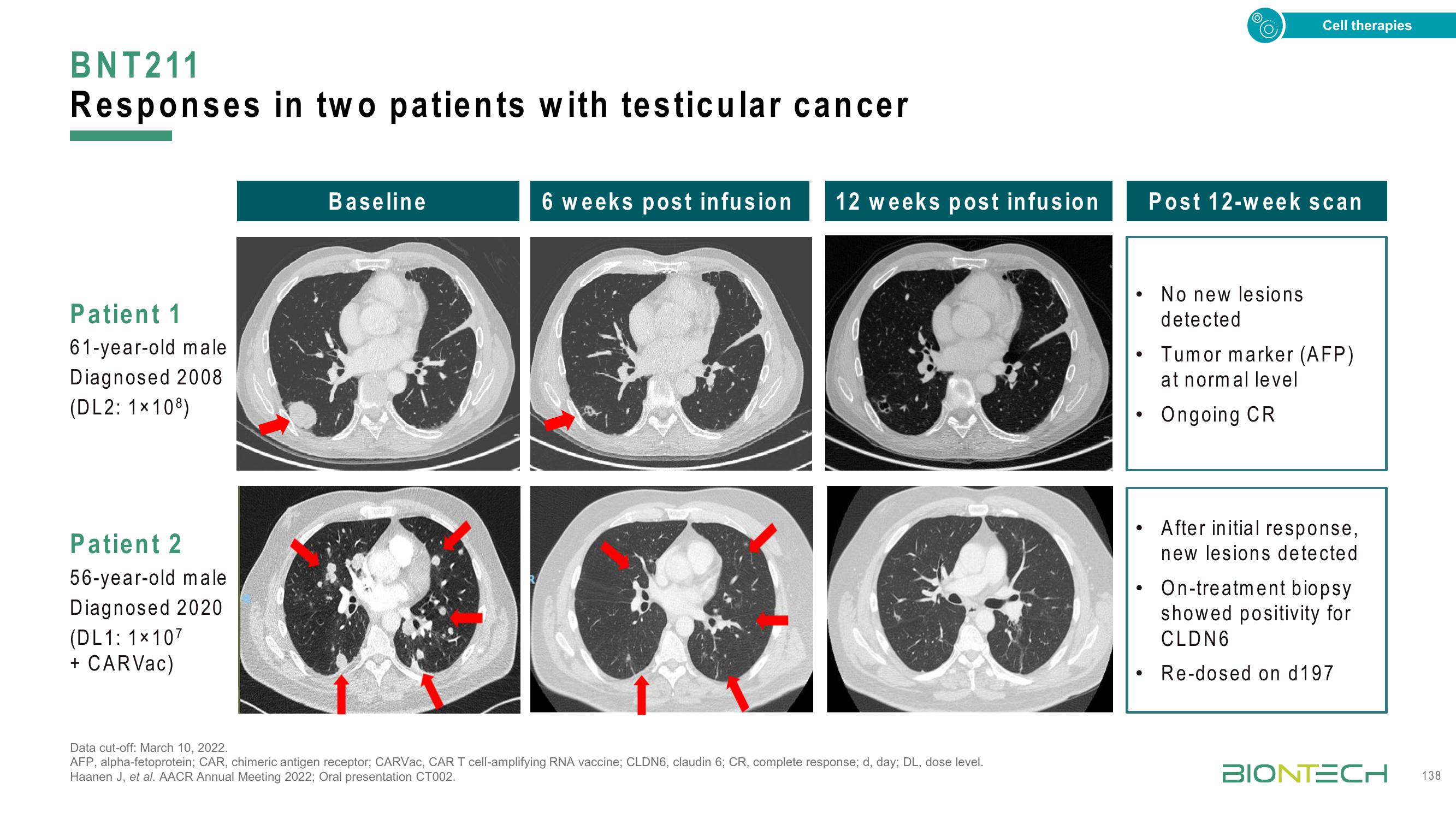
BNT211
Responses in two patients with testicular cancer
Patient 1
61-year-old male
Diagnosed 2008
(DL2: 1×108)
Patient 2
56-year-old male
Diagnosed 2020
(DL1: 1x107
+ CARVac)
Baseline
6 weeks post infusion 12 weeks post infusion
000
OOO
Data cut-off: March 10, 2022.
AFP, alpha-fetoprotein; CAR, chimeric antigen receptor; CARVac, CAR T cell-amplifying RNA vaccine; CLDN6, claudin 6; CR, complete response; d, day; DL, dose level.
Haanen J, et al. AACR Annual Meeting 2022; Oral presentation CT002.
●
●
●
●
●
●
Cell therapies
Post 12-week scan
No new lesions
detected
Tumor marker (AFP)
at normal level
Ongoing CR
After initial response,
new lesions detected
On-treatment biopsy
showed positivity for
CLDN6
Re-dosed on d197
BIONTECH
138View entire presentation