Immix Biopharma Investor Presentation Deck
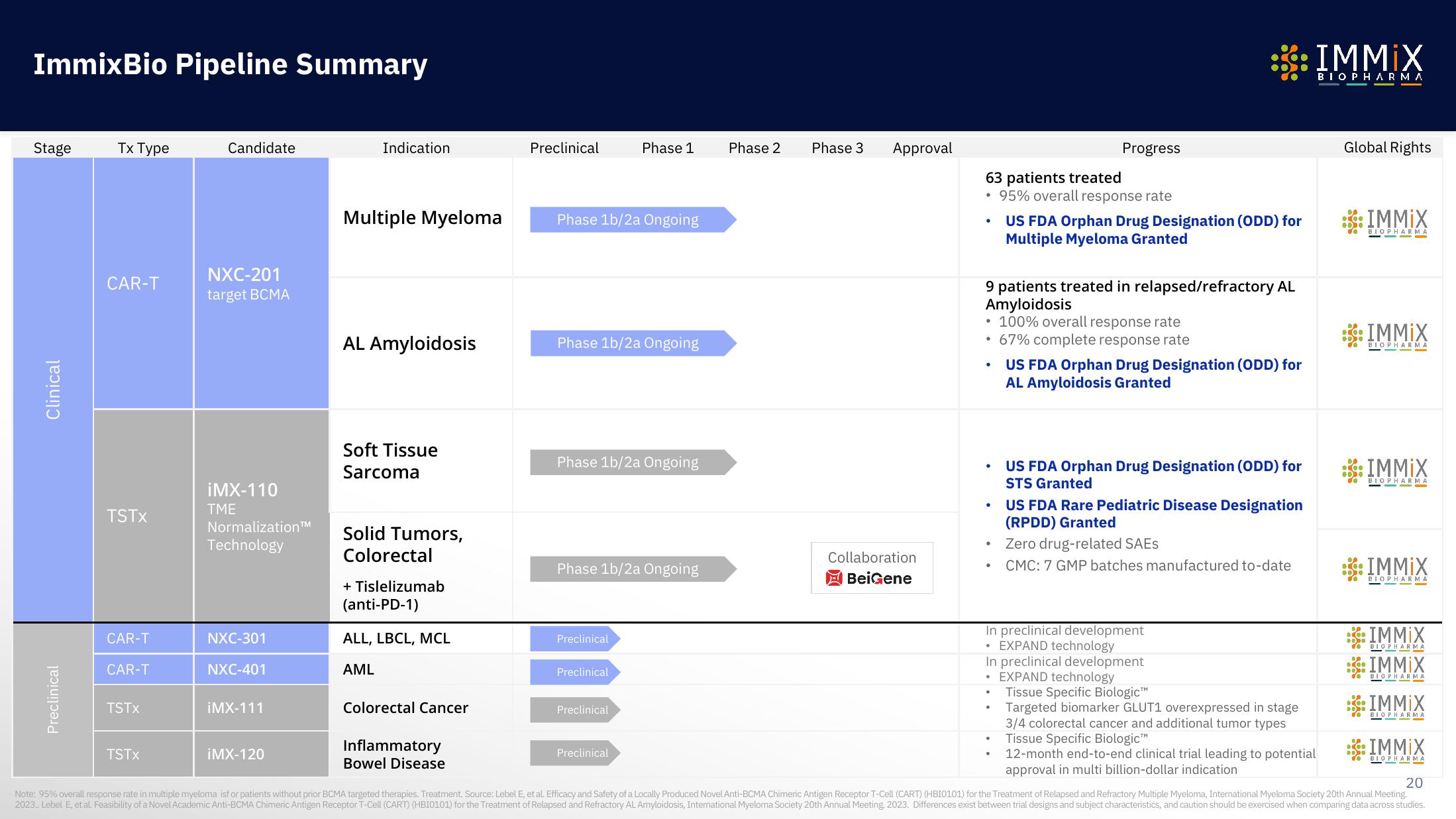
ImmixBio Pipeline Summary
Stage
Clinical
Preclinical
Tx Type
CAR-T
TSTX
CAR-T
CAR-T
TSTX
TSTX
Candidate
NXC-201
target BCMA
¡MX-110
TME
Normalization™
Technology
NXC-301
NXC-401
iMX-111
iMX-120
Indication
Multiple Myeloma
AL Amyloidosis
Soft Tissue
Sarcoma
Solid Tumors,
Colorectal
+ Tislelizumab
(anti-PD-1)
ALL, LBCL, MCL
AML
Colorectal Cancer
Inflammatory
Bowel Disease
Preclinical
Phase 1b/2a Ongoing
Phase 1b/2a Ongoing
Phase 1b/2a Ongoing
Phase 1
Phase 1b/2a Ongoing
Preclinical
Preclinical
Preclinical
Preclinical
Phase 2
Phase 3
Approval
Collaboration
BeiGene
Progress
63 patients treated
95% overall response rate
US FDA Orphan Drug Designation (ODD) for
Multiple Myeloma Granted
.
9 patients treated in relapsed/refractory AL
Amyloidosis
• 100% overall response rate
• 67% complete response rate
.
●●●
IMMIX
S BIOPHARMA
US FDA Orphan Drug Designation (ODD) for
AL Amyloidosis Granted
US FDA Orphan Drug Designation (ODD) for
STS Granted
US FDA Rare Pediatric Disease Designation
(RPDD) Granted
Zero drug-related SAES
CMC: 7 GMP batches manufactured to-date
In preclinical development
EXPAND technology
In preclinical development
EXPAND technology
Tissue Specific Biologic™
Targeted biomarker GLUT1 overexpressed in stage
3/4 colorectal cancer and additional tumor types
Tissue Specific Biologic™
12-month end-to-end clinical trial leading to potential
approval in multi billion-dollar indication
Global Rights
****
+00
do BIOPHARMA
IMMIX
O
IMMIX
...
60 BIOPHARMA
•
+00
•
*00
000
59
•[00
***
sp
00
5
O
00
+00
50
0
***
IMMIX
BIOPHARMA
IMMIX
BIOPHARMA
IMMİX
BIOPHARMA
IMMIX
BIOPHARMA
IMMIX
BIOPHARMA
IMMİX
BIOPHARMA
20
Note: 95% overall response rate in multiple myeloma isf or patients without prior BCMA targeted therapies. Treatment. Source: Lebel E, et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting.
2023.. Lebel E, et al. Feasibility of a Novel Academic Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory AL Amyloidosis, International Myeloma Society 20th Annual Meeting. 2023. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studies.View entire presentation