Equillium Results Presentation Deck
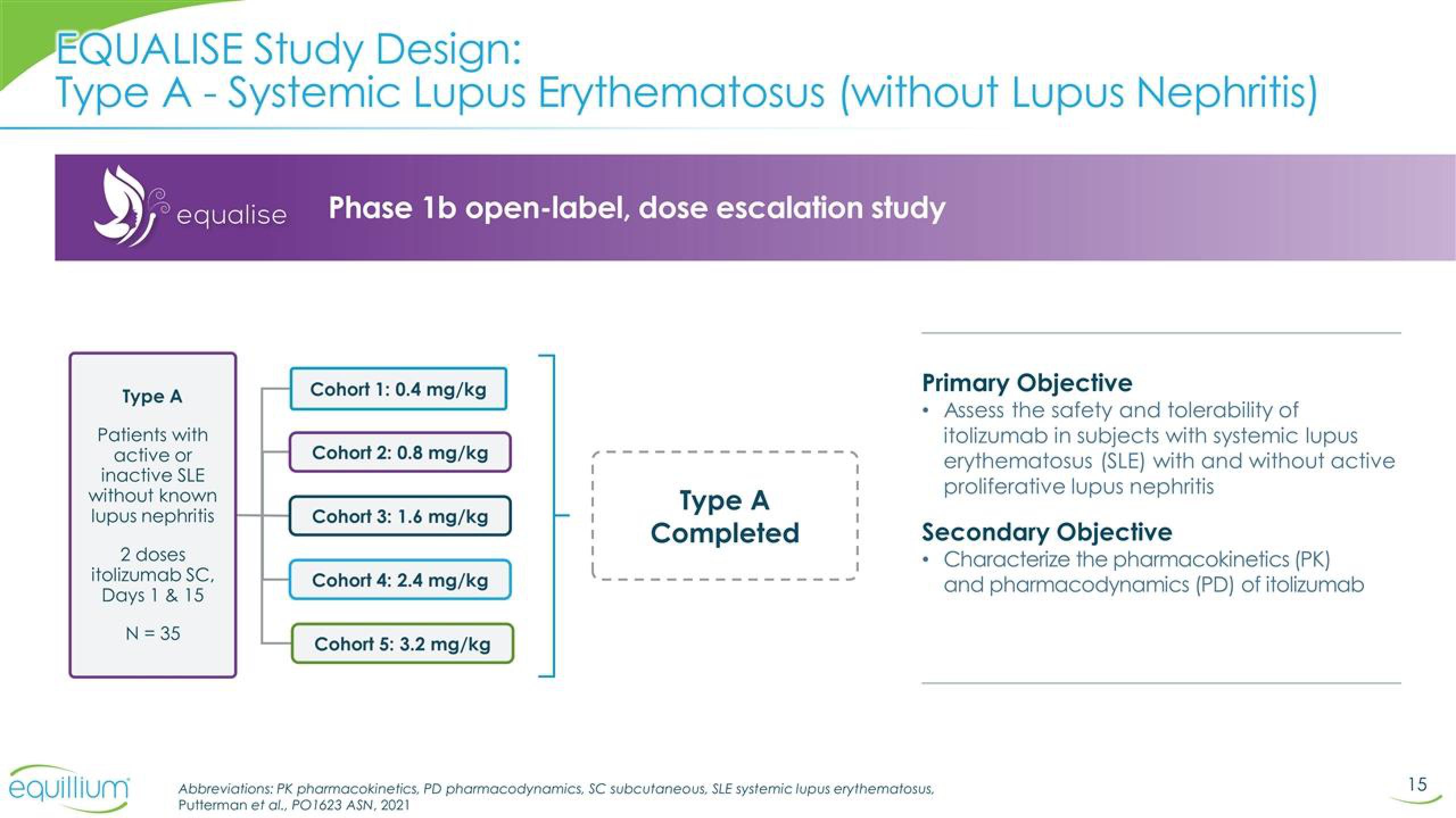
EQUALISE Study Design:
Type A - Systemic Lupus Erythematosus (without Lupus Nephritis)
equalise Phase 1b open-label, dose escalation study
Type A
Patients with
active or
inactive SLE
without known
lupus nephritis
2 doses
itolizumab SC,
Days 1 & 15
N = 35
equillium
Cohort 1: 0.4 mg/kg
Cohort 2: 0.8 mg/kg
Cohort 3: 1.6 mg/kg
Cohort 4: 2.4 mg/kg
Cohort 5: 3.2 mg/kg
Type A
Completed
Primary Objective
• Assess the safety and tolerability of
itolizumab in subjects with systemic lupus
erythematosus (SLE) with and without active
proliferative lupus nephritis
Secondary Objective
• Characterize the pharmacokinetics (PK)
and pharmacodynamics (PD) of itolizumab
Abbreviations: PK pharmacokinetics, PD pharmacodynamics, SC subcutaneous, SLE systemic lupus erythematosus,
Putterman et al., PO1623 ASN, 2021
15View entire presentation