BenevolentAI Investor Day Presentation Deck
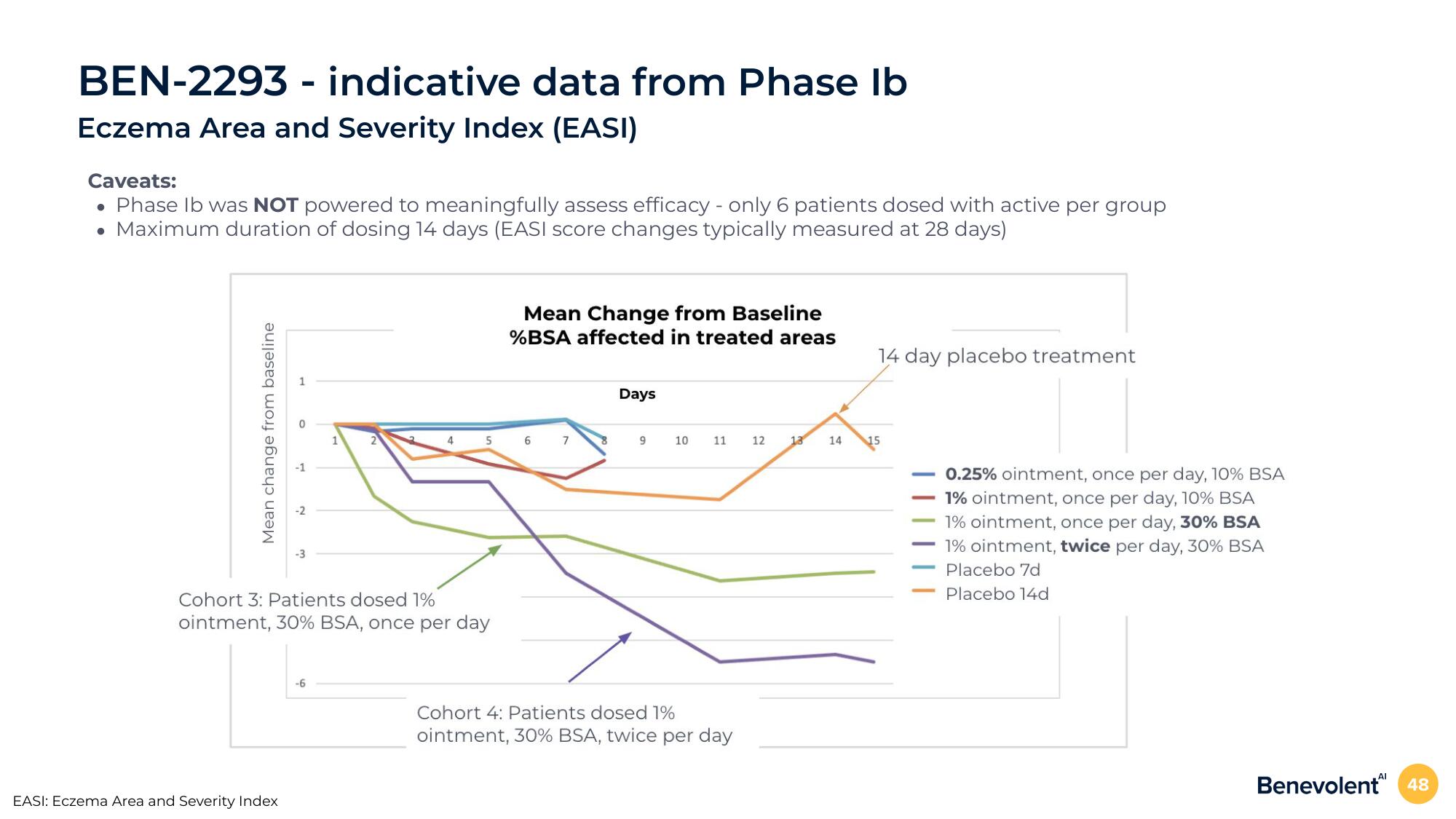
BEN-2293 - indicative data from Phase Ib
Eczema Area and Severity Index (EASI)
Caveats:
• Phase lb was NOT powered to meaningfully assess efficacy - only 6 patients dosed with active per group
• Maximum duration of dosing 14 days (EASI score changes typically measured at 28 days)
Mean change from baseline
-3
EASI: Eczema Area and Severity Index
4
Cohort 3: Patients dosed 1%
ointment, 30% BSA, once per day
-6
5
Mean Change from Baseline
%BSA affected in treated areas
6
7
Days
9
10 11 12 13
Cohort 4: Patients dosed 1%
ointment, 30% BSA, twice per day
14
14 day placebo treatment
15
0.25% ointment, once per day, 10% BSA
1% ointment, once per day, 10% BSA
1% ointment, once per day, 30% BSA
1% ointment, twice per day, 30% BSA
Placebo 7d
Placebo 14d
Benevolent 48View entire presentation