23andMe Investor Presentation Deck
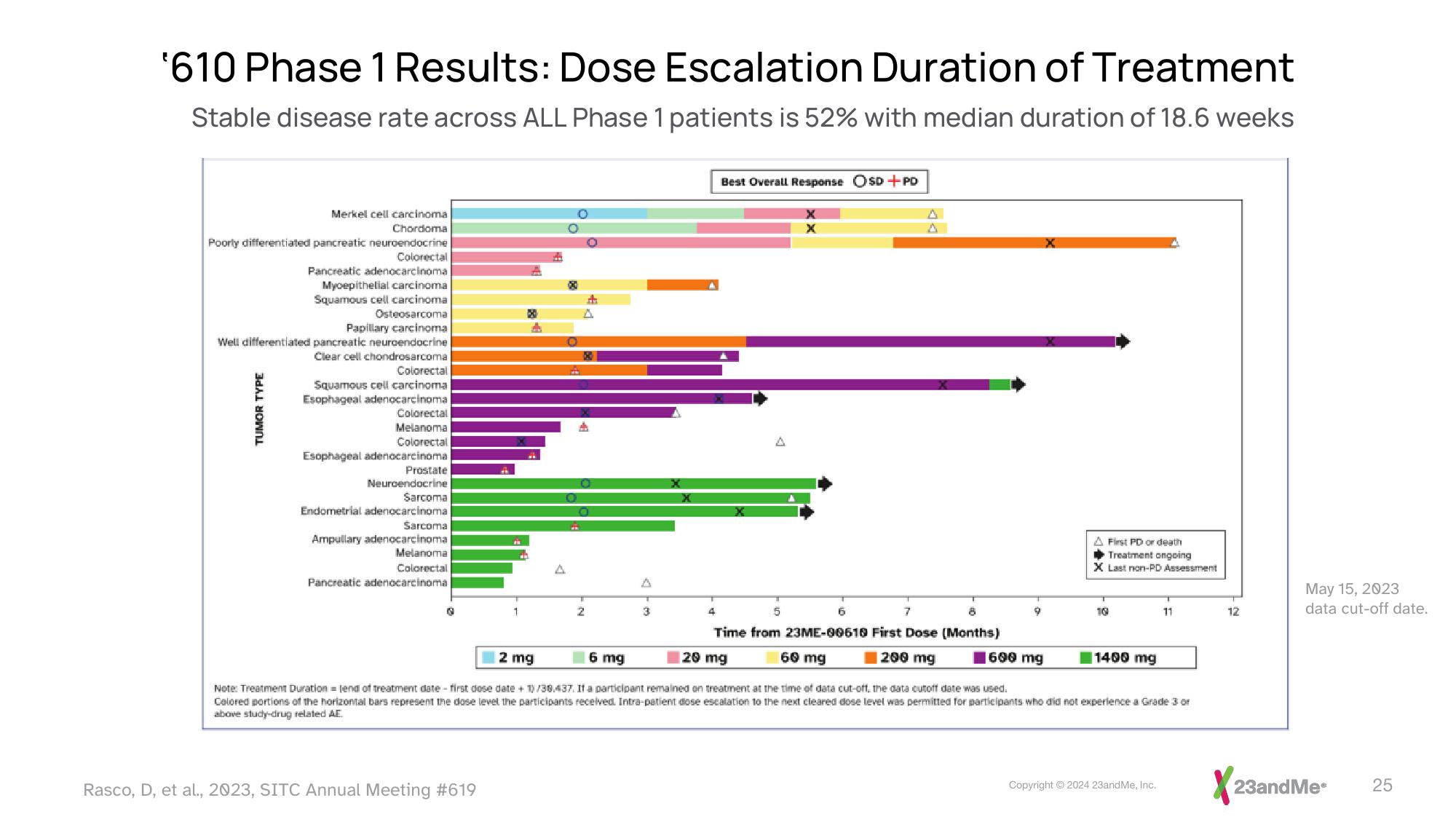
'610 Phase 1 Results: Dose Escalation Duration of Treatment
Stable disease rate across ALL Phase 1 patients is 52% with median duration of 18.6 weeks
Merkel cell carcinoma.
Chordoma
Poorly differentiated pancreatic neuroendocrine
Colorectal
Pancreatic adenocarcinoma
Myoepithelial carcinoma
Squamous cell carcinoma
TUMOR TYPE
Osteosarcoma
Papillary carcinoma
Well differentiated pancreatic neuroendocrine
Clear cell chondrosarcoma
Colorectal
Squamous cell carcinoma.
Esophageal adenocarcinoma
Colorectal
Melanoma
Colorectal
Esophageal adenocarcinoma
Prostate
Neuroendocrine
Sarcoma
Endometrial adenocarcinoma
Sarcoma
Ampullary adenocarcinoma
Melanoma
Colorectal
Pancreatic adenocarcinoma
10
A
Rasco, D, et al., 2023, SITC Annual Meeting #619
2
A
3
Best Overall Response OSD+PD
4
5
8
Time from 23ME-00610 First Dose (Months)
60 mg
200 mg
6
20 mg
7
9
A First PD or death
Treatment ongoing
X Last non-PD Assessment
10
600 mg
2 mg
6 mg
Note: Treatment Duration=lend of treatment date first dose date + 1)/38.437. If a participant remained on treatment at the time of data cut-off, the data cutoff date was used.
Colored portions of the horizontal bars represent the dose level the participants received. Intra-patient dose escalation to the next cleared dose level was permitted for participants who did not experience a Grade 3 or
above study-drug related AE
1400 mg
11
Copyright © 2024 23andMe, Inc.
12
May 15, 2023
data cut-off date.
23andMe 25View entire presentation