BioNTech Results Presentation Deck
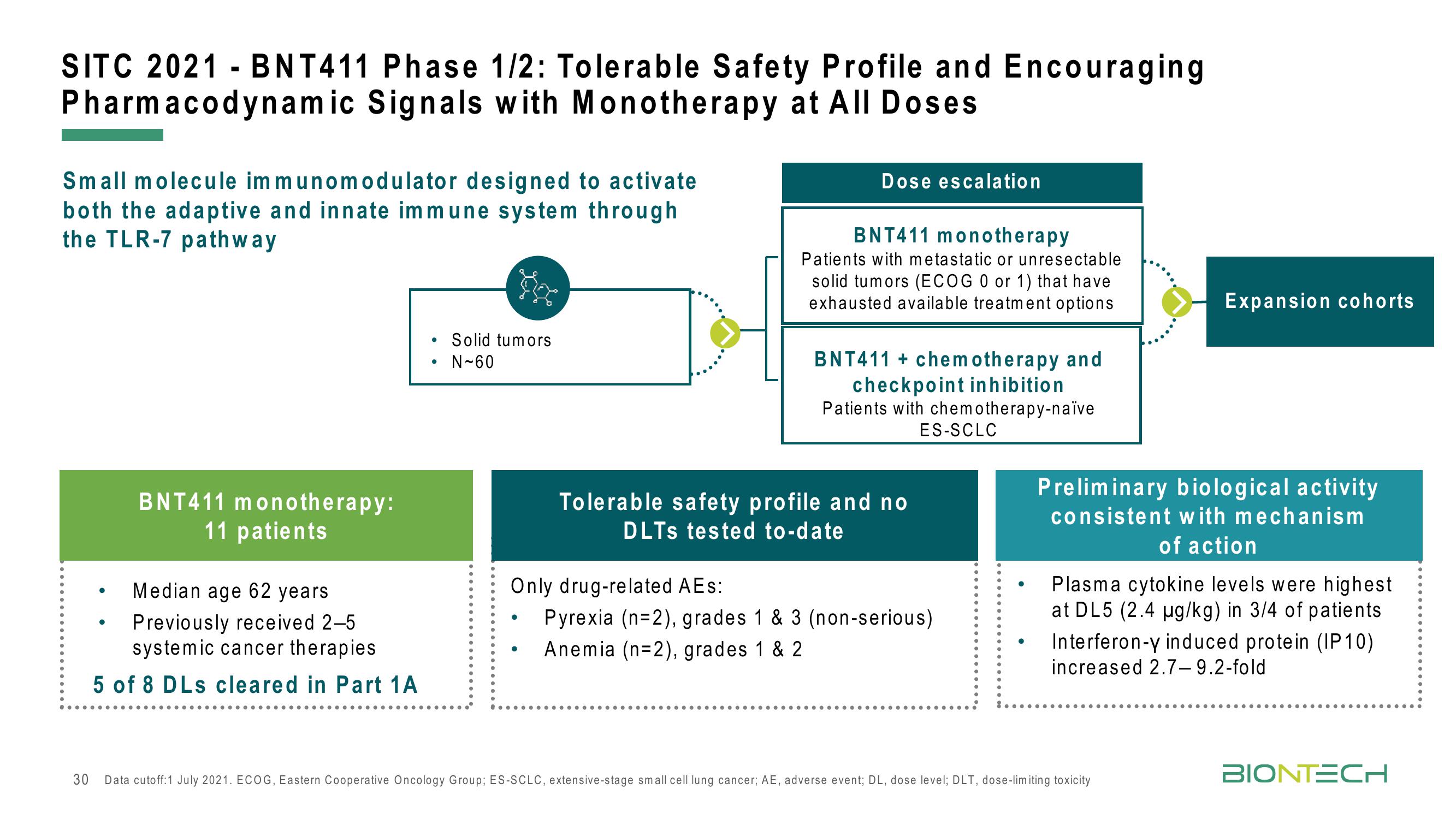
SITC 2021 - BNT411 Phase 1/2: Tolerable Safety Profile and Encouraging
Pharmacodynamic Signals with Monotherapy at All Doses
Small molecule immunomodulator designed to activate
both the adaptive and innate immune system through
the TLR-7 pathway
30
BNT411 monotherapy:
11 patients
Median age 62 years
Previously received 2-5
systemic cancer therapies
5 of 8 DLs cleared in Part 1 A
●
Solid tumors
N-60
Dose escalation
●
BNT411 monotherapy
Patients with metastatic or unresectable
solid tumors (ECOG 0 or 1) that have
exhausted available treatment options
BNT411 + chemotherapy and
checkpoint inhibition
Patients with chemotherapy-naïve
Tolerable safety profile and no
DLTS tested to-date
ES-SCLC
Only drug-related AEs:
Pyrexia (n=2), grades 1 & 3 (non-serious)
Anemia (n=2), grades 1 & 2
Expansion cohorts
Preliminary biological activity
consistent with mechanism
of action
Plasma cytokine levels were highest.
at DL5 (2.4 µg/kg) in 3/4 of patients
Interferon-y induced protein (IP10)
increased 2.7-9.2-fold
Data cutoff:1 July 2021. ECOG, Eastern Cooperative Oncology Group; ES-SCLC, extensive-stage small cell lung cancer; AE, adverse event; DL, dose level; DLT, dose-limiting toxicity
BIONTECHView entire presentation