Ocuphire Pharma Investor Presentation Deck
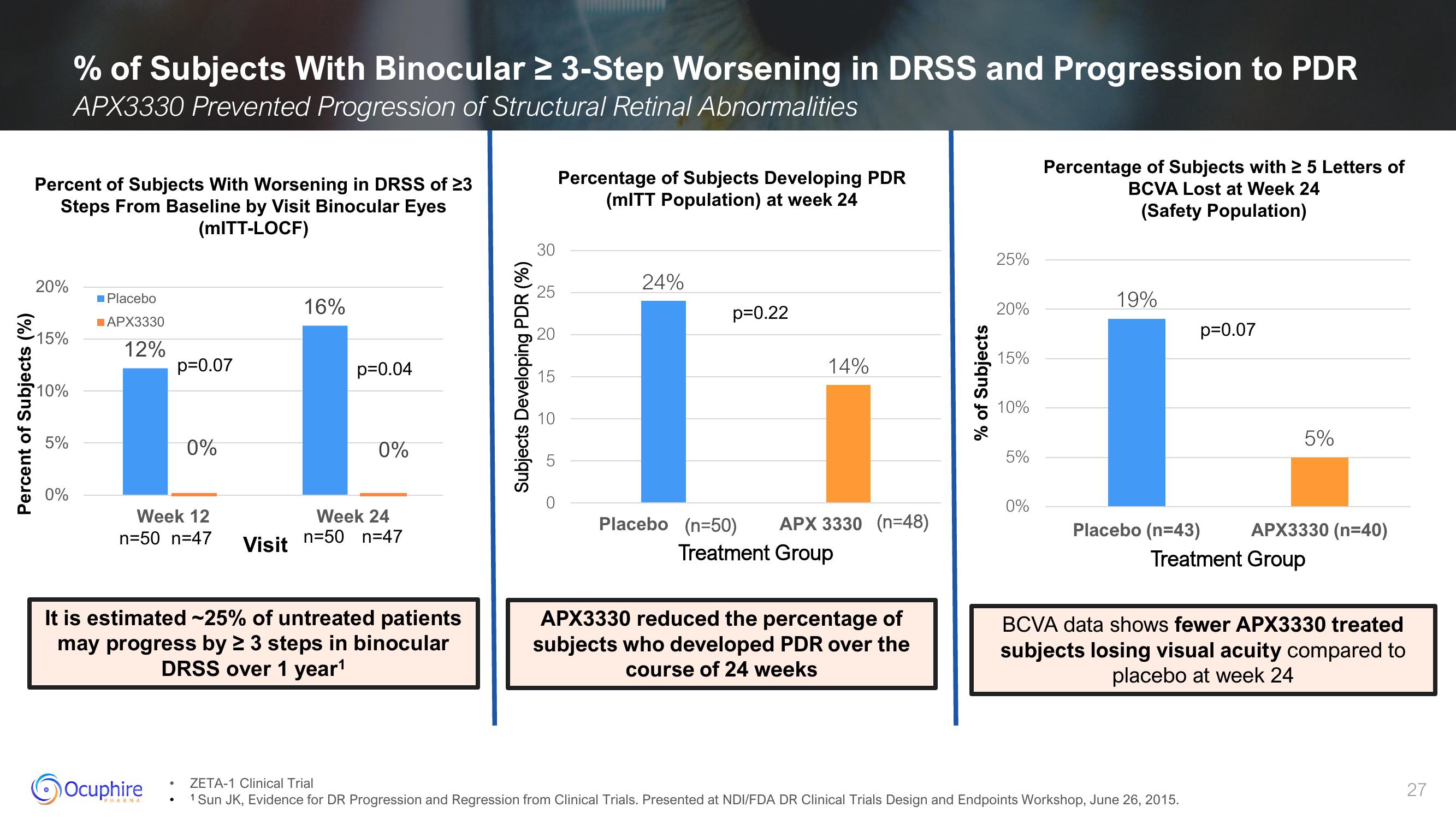
% of Subjects With Binocular ≥ 3-Step Worsening in DRSS and Progression to PDR
APX3330 Prevented Progression of Structural Retinal Abnormalities
Percent of Subjects With Worsening in DRSS of 23
Steps From Baseline by Visit Binocular Eyes
(mITT-LOCF)
20%
■Placebo
APX3330
15%
12%
p=0.07
10%
D
5%
0%
0%
Week 12
n=50 n=47
Ocuphire
Visit
●
16%
p=0.04
0%
It is estimated ~25% of untreated patients
may progress by ≥ 3 steps in binocular
DRSS over 1 year¹
Week 24
n=50 n=47
30
25
20
15
10
Percentage of Subjects Developing PDR
(mITT Population) at week 24
24%
p=0.22
14%
Placebo (n=50)
APX 3330 (n=48)
Treatment Group
APX3330 reduced the percentage of
subjects who developed PDR over the
course of 24 weeks
25%
20%
15%
10%
5%
0%
Percentage of Subjects with ≥ 5 Letters of
BCVA Lost at Week 24
(Safety Population)
19%
p=0.07
Placebo (n=43)
APX3330 (n=40)
Treatment Group
5%
ZETA-1 Clinical Trial
1 Sun JK, Evidence for DR Progression and Regression from Clinical Trials. Presented at NDI/FDA DR Clinical Trials Design and Endpoints Workshop, June 26, 2015.
BCVA data shows fewer APX3330 treated
subjects losing visual acuity compared to
placebo at week 24
27View entire presentation