Neumora Therapeutics IPO Presentation Deck
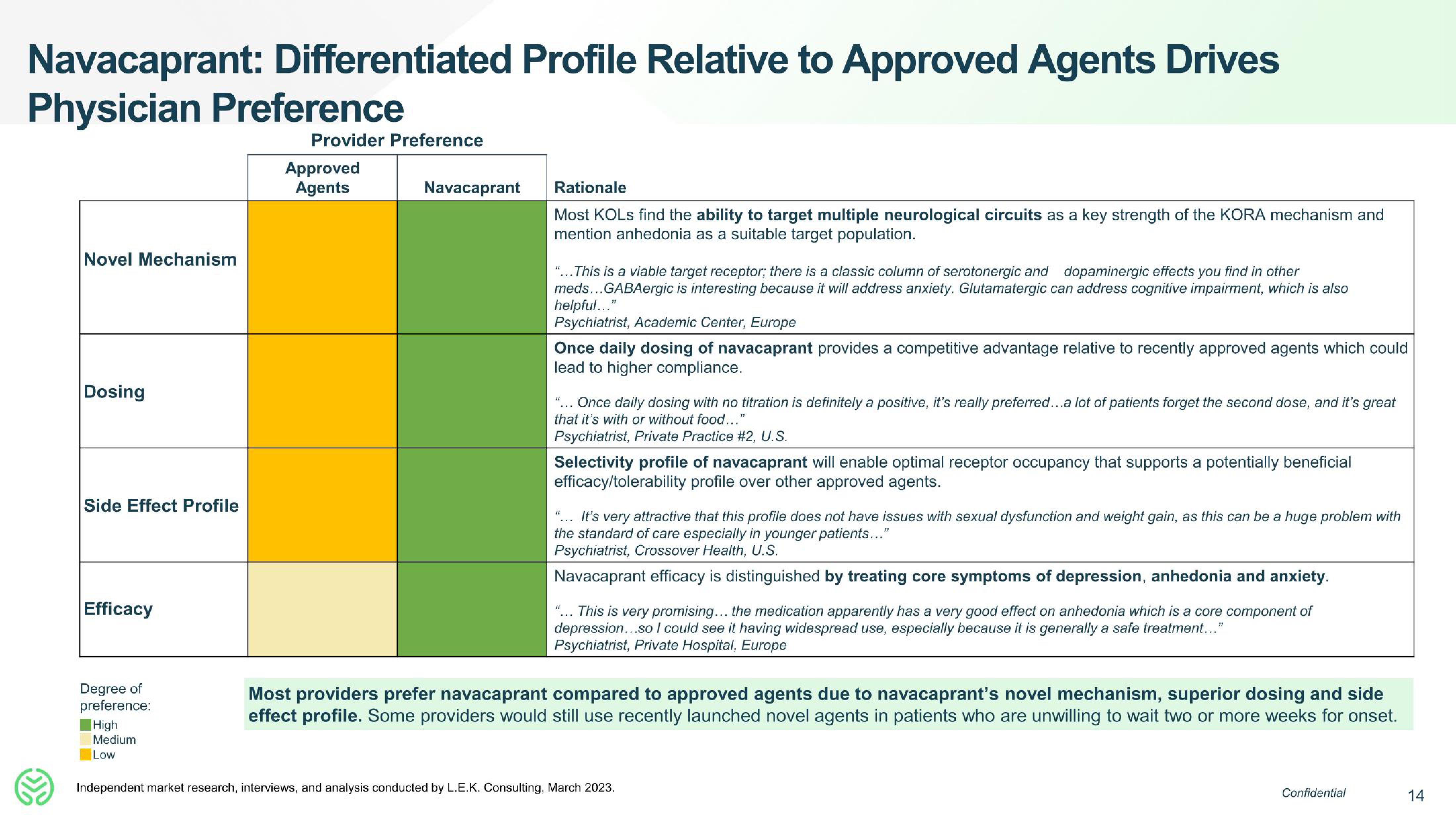
Navacaprant: Differentiated Profile Relative to Approved Agents Drives
Physician Preference
Novel Mechanism
Dosing
Side Effect Profile
Efficacy
Degree of
preference:
High
Medium
Low
Provider Preference
Approved
Agents
Navacaprant
Rationale
Most KOLs find the ability to target multiple neurological circuits as a key strength of the KORA mechanism and
mention anhedonia as a suitable target population.
"...This is a viable target receptor; there is a classic column of serotonergic and dopaminergic effects you find in other
meds... GABAergic is interesting because it will address anxiety. Glutamatergic can address cognitive impairment, which is also
helpful..."
Psychiatrist, Academic Center, Europe
Once daily dosing of navacaprant provides a competitive advantage relative to recently approved agents which could
lead to higher compliance.
"... Once daily dosing with no titration is definitely a positive, it's really preferred...a lot of patients forget the second dose, and it's great
that it's with or without food..."
Psychiatrist, Private Practice #2, U.S.
Selectivity profile of navacaprant will enable optimal receptor occupancy that supports a potentially beneficial
efficacy/tolerability profile over other approved agents.
"... It's very attractive that this profile does not have issues with sexual dysfunction and weight gain, as this can be a huge problem with
the standard of care especially in younger patients..."
Psychiatrist, Crossover Health, U.S.
Navacaprant efficacy is distinguished by treating core symptoms of depression, anhedonia and anxiety.
"... This is very promising... the medication apparently has a very good effect on anhedonia which is a core component of
depression...so I could see it having widespread use, especially because it is generally a safe treatment..."
Psychiatrist, Private Hospital, Europe
Most providers prefer navacaprant compared to approved agents due to navacaprant's novel mechanism, superior dosing and side
effect profile. Some providers would still use recently launched novel agents in patients who are unwilling to wait two or more weeks for onset.
Independent market research, interviews, and analysis conducted by L.E.K. Consulting, March 2023.
Confidential
14View entire presentation