Investor Presentation
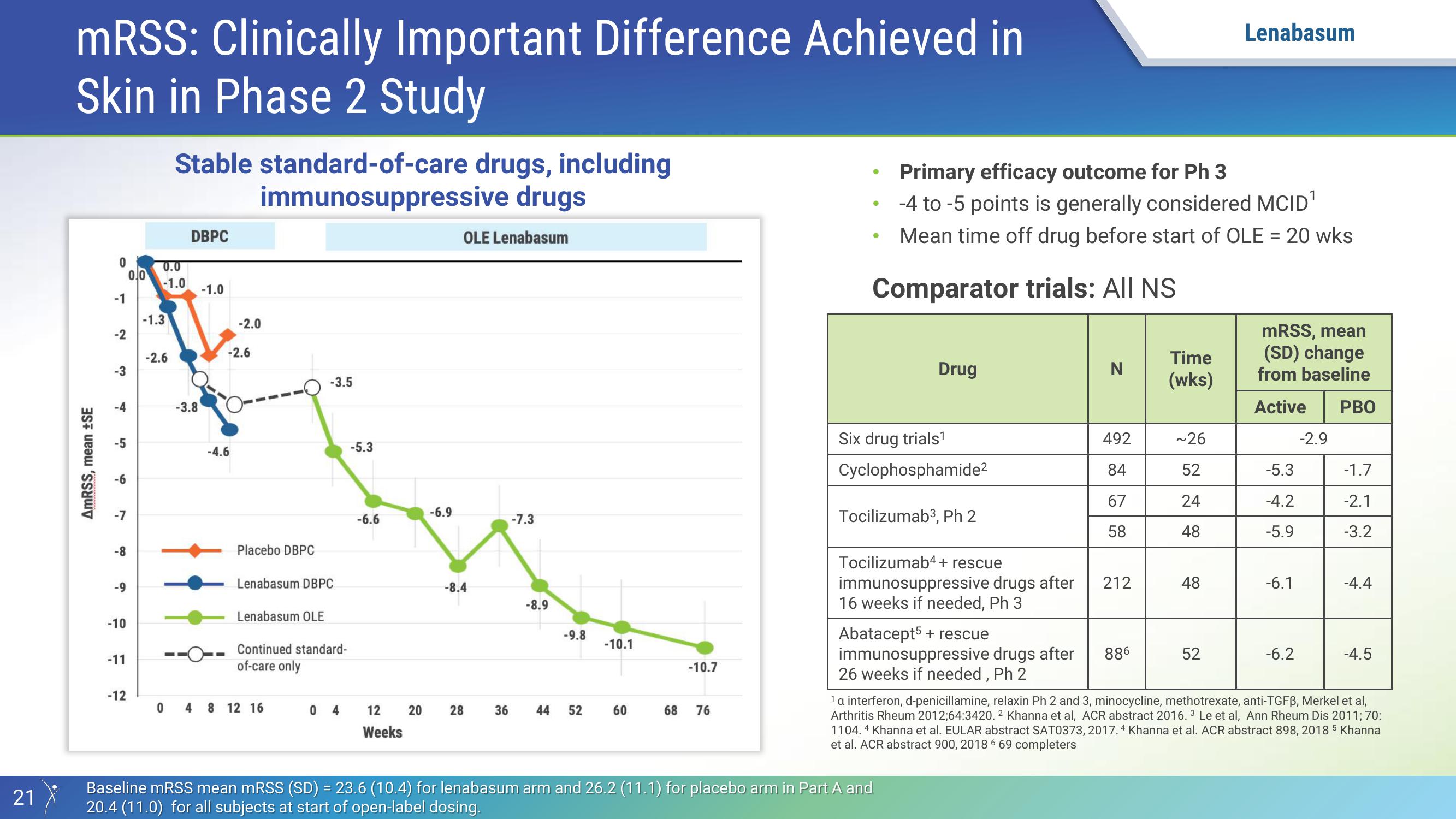
mRSS: Clinically Important Difference Achieved in
Skin in Phase 2 Study
AmRSS, mean +SE
0
-1
-2
-3
T
5
6
7
-8
-9
-10
-11
-12
0,0
0.0
-1.0
-1.3
Stable standard-of-care drugs, including
immunosuppressive drugs
-2.6
DBPC
-3.8
-1.0
-2.0
-2.6
-4.6
Placebo DBPC
Lenabasum DBPC
Lenabasum OLE
-3.5
Continued standard-
of-care only
0 4 8 12 16
0
4
-5.3
-6.6
12
Weeks
20
-6.9
OLE Lenabasum
-8.4
28
-7.3
-8.9
-9.8
36 44 52
-10.1
60
-10.7
68 76
Primary efficacy outcome for Ph 3
-4 to -5 points is generally considered MCID¹
Mean time off drug before start of OLE = 20 wks
Comparator trials: All NS
●
●
Drug
Six drug trials¹
Cyclophosphamide²
Tocilizumab3, Ph 2
Tocilizumab4+ rescue
immunosuppressive drugs after
16 weeks if needed, Ph 3
Abatacept5 + rescue
immunosuppressive drugs after
26 weeks if needed, Ph 2
21 X
Baseline mRSS mean mRSS (SD) = 23.6 (10.4) for lenabasum arm and 26.2 (11.1) for placebo arm in Part A and
20.4 (11.0) for all subjects at start of open-label dosing.
N
492
84
67
58
212
886
Time
(wks)
~26
52
24
48
48
Lenabasum
52
mRSS, mean
(SD) change
from baseline
Active PBO
-5.3
-4.2
-5.9
-6.1
-6.2
-2.9
-1.7
-2.1
-3.2
-4.4
-4.5
a interferon, d-penicillamine, relaxin Ph 2 and 3, minocycline, methotrexate, anti-TGFB, Merkel et al,
Arthritis Rheum 2012;64:3420. ² Khanna et al, ACR abstract 2016. ³ Le et al, Ann Rheum Dis 2011; 70:
1104. 4 Khanna et al. EULAR abstract SAT0373, 2017. 4 Khanna et al. ACR abstract 898, 2018 5 Khanna
et al. ACR abstract 900, 2018 6 69 completersView entire presentation