Ocuphire Pharma Results
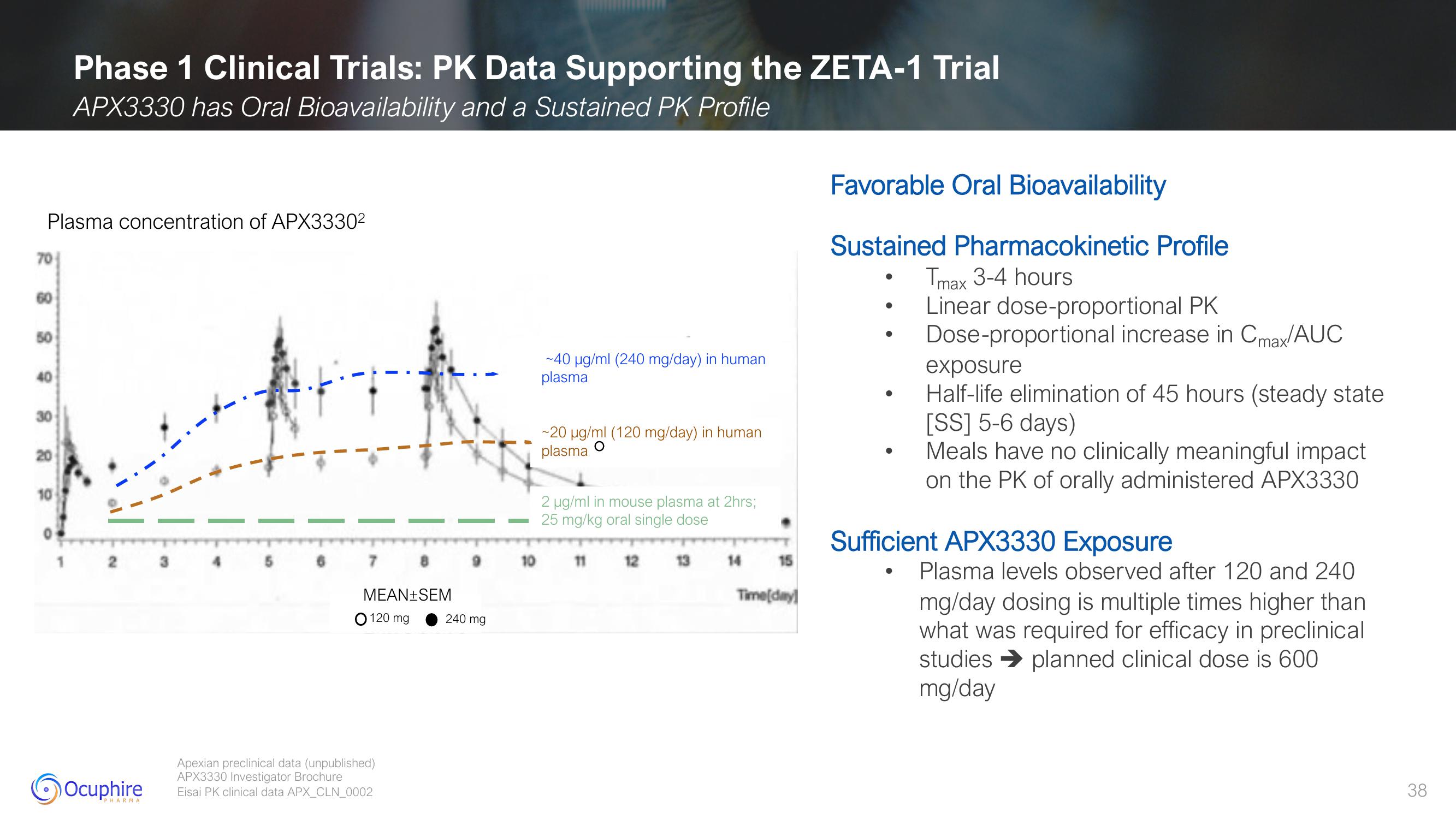
Plasma concentration of APX3330²
70
60
50
40
30
20
10
Phase 1 Clinical Trials: PK Data Supporting the ZETA-1 Trial
APX3330 has Oral Bioavailability and a Sustained PK Profile
1
2 3
Ocuphire
PHARMA
MEAN+SEM
O120 mg
Apexian preclinical data (unpublished)
APX3330 Investigator Brochure
Eisai PK clinical data APX_CLN_0002
240 mg
~40 µg/ml (240 mg/day) in human
plasma
~20 µg/ml (120 mg/day) in human
O
plasma
2 µg/ml in mouse plasma at 2hrs;
25 mg/kg oral single dose
10 11 12 13
14
15
Time[day]
Favorable Oral Bioavailability
Sustained Pharmacokinetic Profile
Tmax 3-4 hours
Linear dose-proportional PK
Dose-proportional increase in Cmax/AUC
exposure
Half-life elimination of 45 hours (steady state
[SS] 5-6 days)
Meals have no clinically meaningful impact
on the PK of orally administered APX3330
Sufficient APX3330 Exposure
Plasma levels observed after 120 and 240
mg/day dosing is multiple times higher than
what was required for efficacy in preclinical
studies planned clinical dose is 600
mg/day
38View entire presentation