AstraZeneca Results Presentation Deck
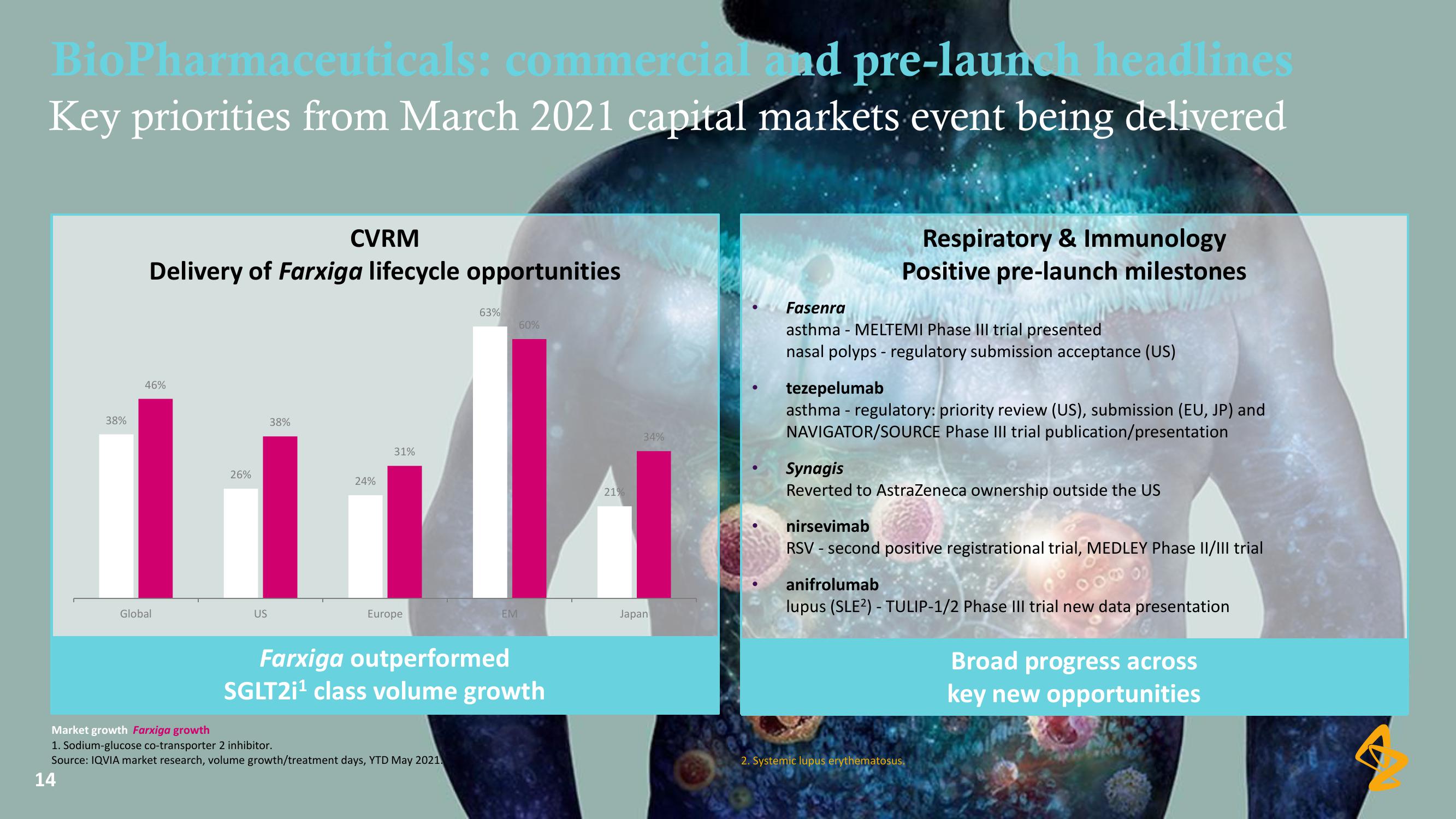
BioPharmaceuticals: commercial and pre-launch headlines
Key priorities from March 2021 capital markets event being delivered
38%
CVRM
Delivery of Farxiga lifecycle opportunities
46%
Global
26%
US
38%
31%
24%
1
Europe
63%
Market growth Farxiga growth
1. Sodium-glucose co-transporter 2 inhibitor.
Source: IQVIA market research, volume growth/treatment days, YTD May 2021.
14
EM
60%
Farxiga outperformed
SGLT2i¹ class volume growth
21%
34%
Japan
Respiratory & Immunology
Positive pre-launch milestones
20
Fasenra
asthma - MELTEMI Phase III trial presented
enraa
nasal polyps - regulatory submission acceptance (US)
tezepelumab
asthma - regulatory: priority review (US), submission (EU, JP) and
NAVIGATOR/SOURCE Phase III trial publication/presentation
Synagis
Reverted to AstraZeneca ownership outside the US
nirsevimab
RSV - second positive registrational trial, MEDLEY Phase II/III trial
anifrolumab
lupus (SLE²) - TULIP-1/2 Phase III trial new data presentation
2. Systemic lupus erythematosus.
Broad progress across
key new opportunitiesView entire presentation