Kymera Investor Presentation Deck
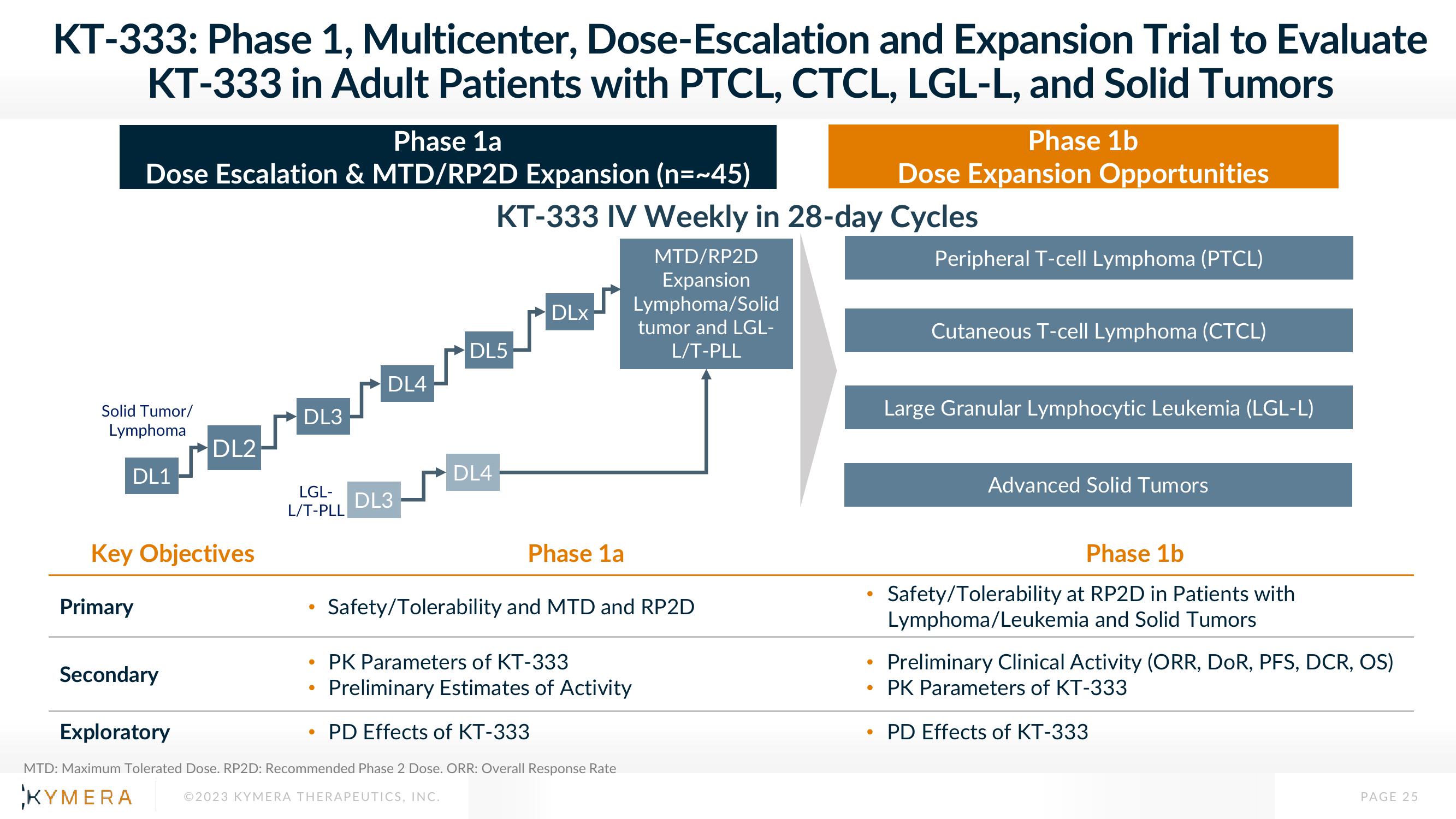
KT-333: Phase 1, Multicenter, Dose-Escalation and Expansion Trial to Evaluate
KT-333 in Adult Patients with PTCL, CTCL, LGL-L, and Solid Tumors
Phase 1b
Dose Expansion Opportunities
Phase 1a
Dose Escalation & MTD/RP2D Expansion (n=~45)
Solid Tumor/
Lymphoma
DL1
Primary
Key Objectives
Secondary
DL2
Exploratory
DL3
LGL-
L/T-PLL
●
●
●
●
DL4
DL3
KT-333 IV Weekly in 28-day Cycles
DL5
DL4
DLX
Phase 1a
Safety/Tolerability and MTD and RP2D
PK Parameters of KT-333
Preliminary Estimates of Activity
PD Effects of KT-333
MTD/RP2D
Expansion
Lymphoma/Solid
tumor and LGL-
L/T-PLL
MTD: Maximum Tolerated Dose. RP2D: Recommended Phase 2 Dose. ORR: Overall Response Rate
KYMERA
Ⓒ2023 KYMERA THERAPEUTICS, INC.
●
●
●
Peripheral T-cell Lymphoma (PTCL)
Cutaneous T-cell Lymphoma (CTCL)
Large Granular Lymphocytic Leukemia (LGL-L)
Advanced Solid Tumors
Phase 1b
Safety/Tolerability at RP2D in Patients with
Lymphoma/Leukemia and Solid Tumors
Preliminary Clinical Activity (ORR, DOR, PFS, DCR, OS)
PK Parameters of KT-333
PD Effects of KT-333
PAGE 25View entire presentation