Company Overview
Ajuo esn
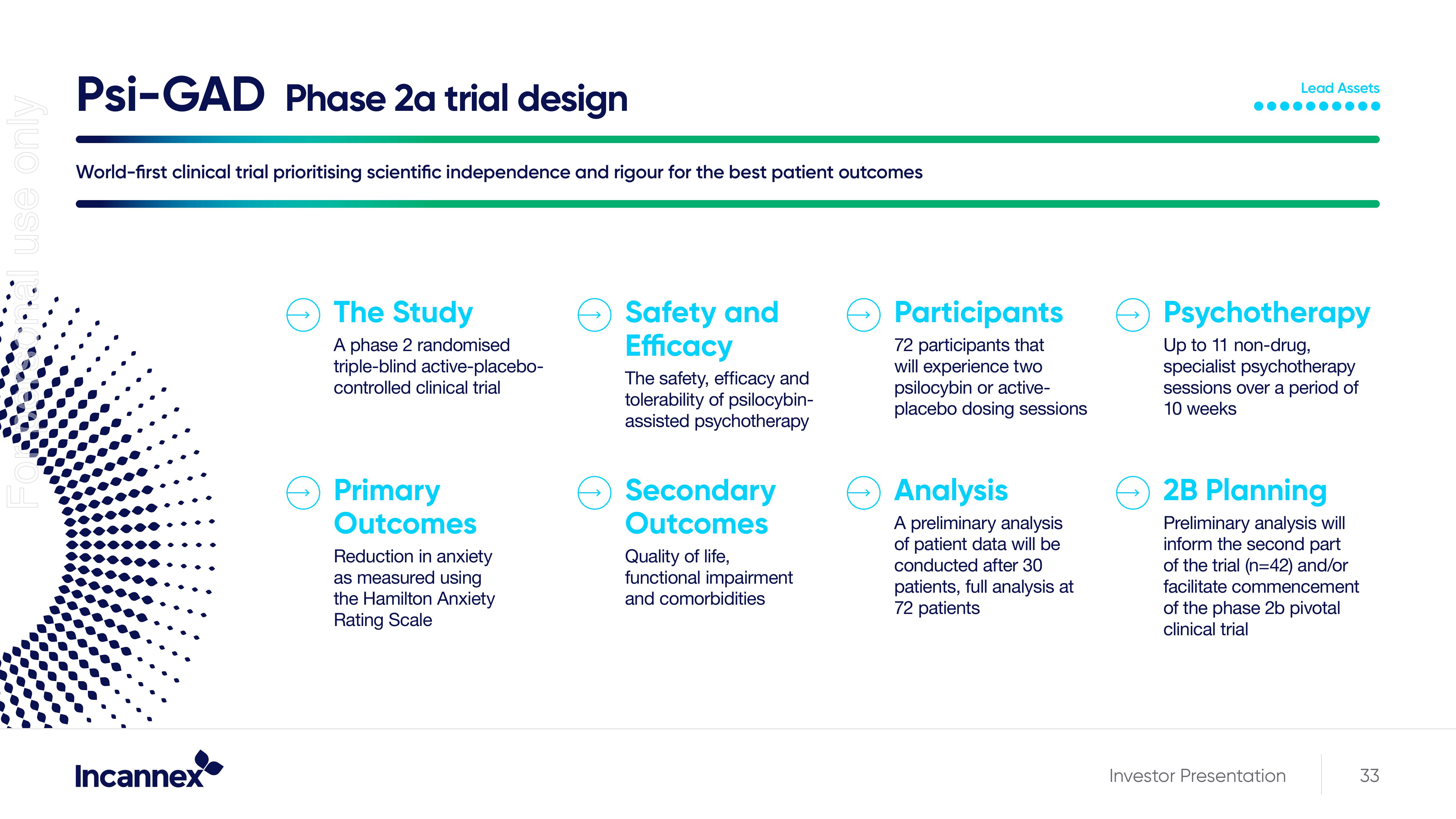
Psi-GAD Phase 2a trial design
World-first clinical trial prioritising scientific independence and rigour for the best patient outcomes
Incannex
The Study
A phase 2 randomised
triple-blind active-placebo-
controlled clinical trial
Primary
Outcomes
Reduction in anxiety
as measured using
the Hamilton Anxiety
Rating Scale
Safety and
Efficacy
The safety, efficacy and
tolerability of psilocybin-
assisted psychotherapy
Secondary
Outcomes
Quality of life,
functional impairment
and comorbidities
Participants
72 participants that
will experience two
psilocybin or active-
placebo dosing sessions
Analysis
A preliminary analysis
of patient data will be
conducted after 30
patients, full analysis at
72 patients
Lead Assets
●●●●●●●●●●
Psychotherapy
Up to 11 non-drug,
specialist psychotherapy
sessions over a period of
10 weeks
2B Planning
Preliminary analysis will
inform the second part
of the trial (n=42) and/or
facilitate commencement
of the phase 2b pivotal
clinical trial
Investor Presentation
33View entire presentation