AstraZeneca Investor Day Presentation Deck
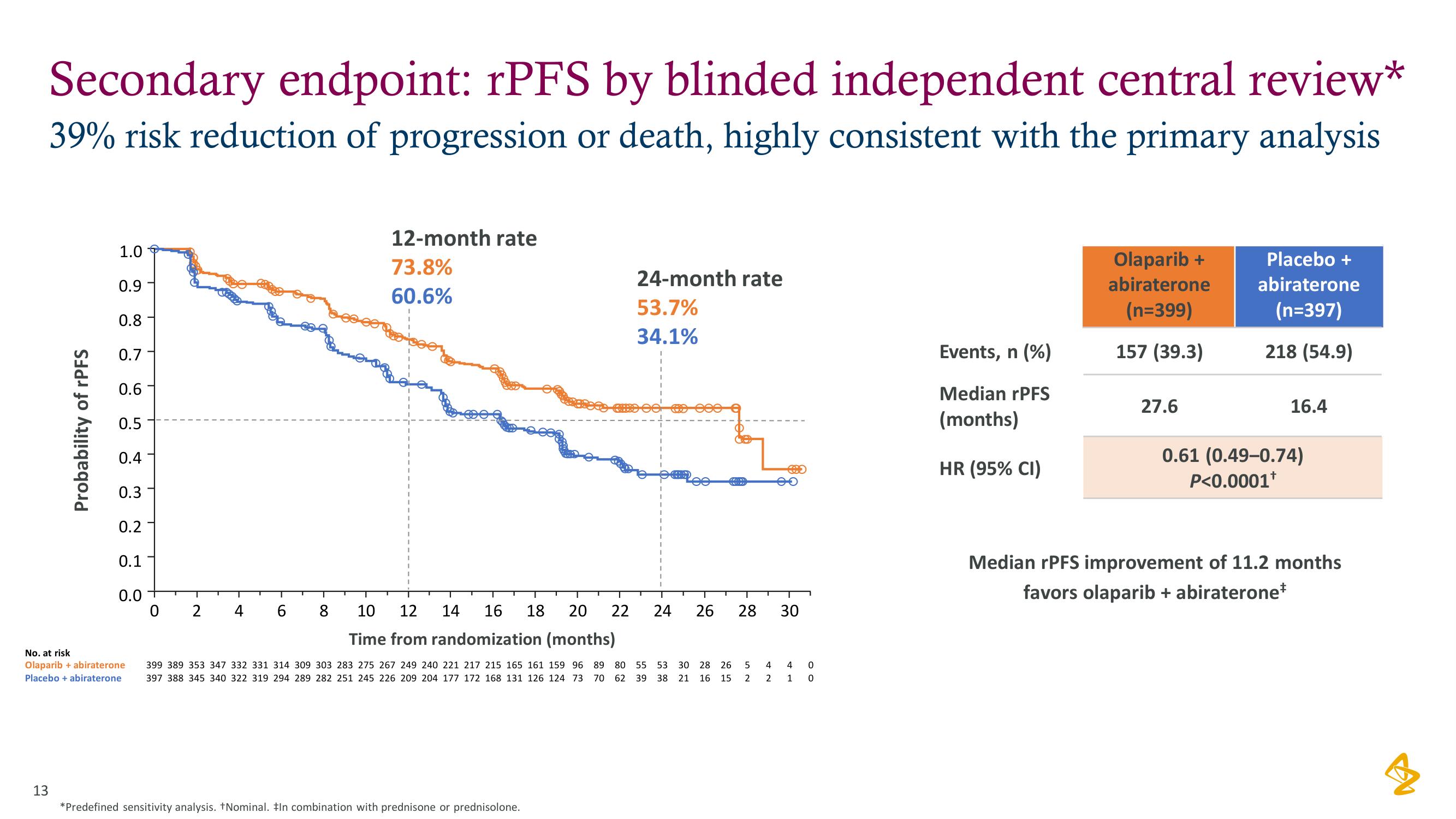
Secondary endpoint: rPFS by blinded independent central review*
39% risk reduction of progression or death, highly consistent with the primary analysis
Probability of rPFS
13
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
No. at risk
Olaparib + abiraterone
Placebo + abiraterone
0
2
4
10 12 14 16 18 20
Time from randomization (months)
399 389 353 347 332 331 314 309 303 283 275 267 249 240 221 217 215 165 161 159 96 89 80 55 53 30 28 26 5
397 388 345 340 322 319 294 289 282 251 245 226 209 204 177 172 168 131 126 124 73 70 62 39 38 21 16 15 2
6
12-month rate
73.8%
60.6%
8
24-month rate
53.7%
34.1%
*Predefined sensitivity analysis. +Nominal. In combination with prednisone or prednisolone.
900 6 of
22 24
26 28
4
2
30
4
1
o o
0
0
Events, n (%)
Median rPFS
(months)
HR (95% CI)
Olaparib +
abiraterone
(n=399)
157 (39.3)
27.6
Placebo +
abiraterone
(n=397)
218 (54.9)
16.4
0.61 (0.49-0.74)
P<0.0001¹
Median rPFS improvement of 11.2 months
favors olaparib + abiraterone*
BView entire presentation