MaxCyte IPO Presentation Deck
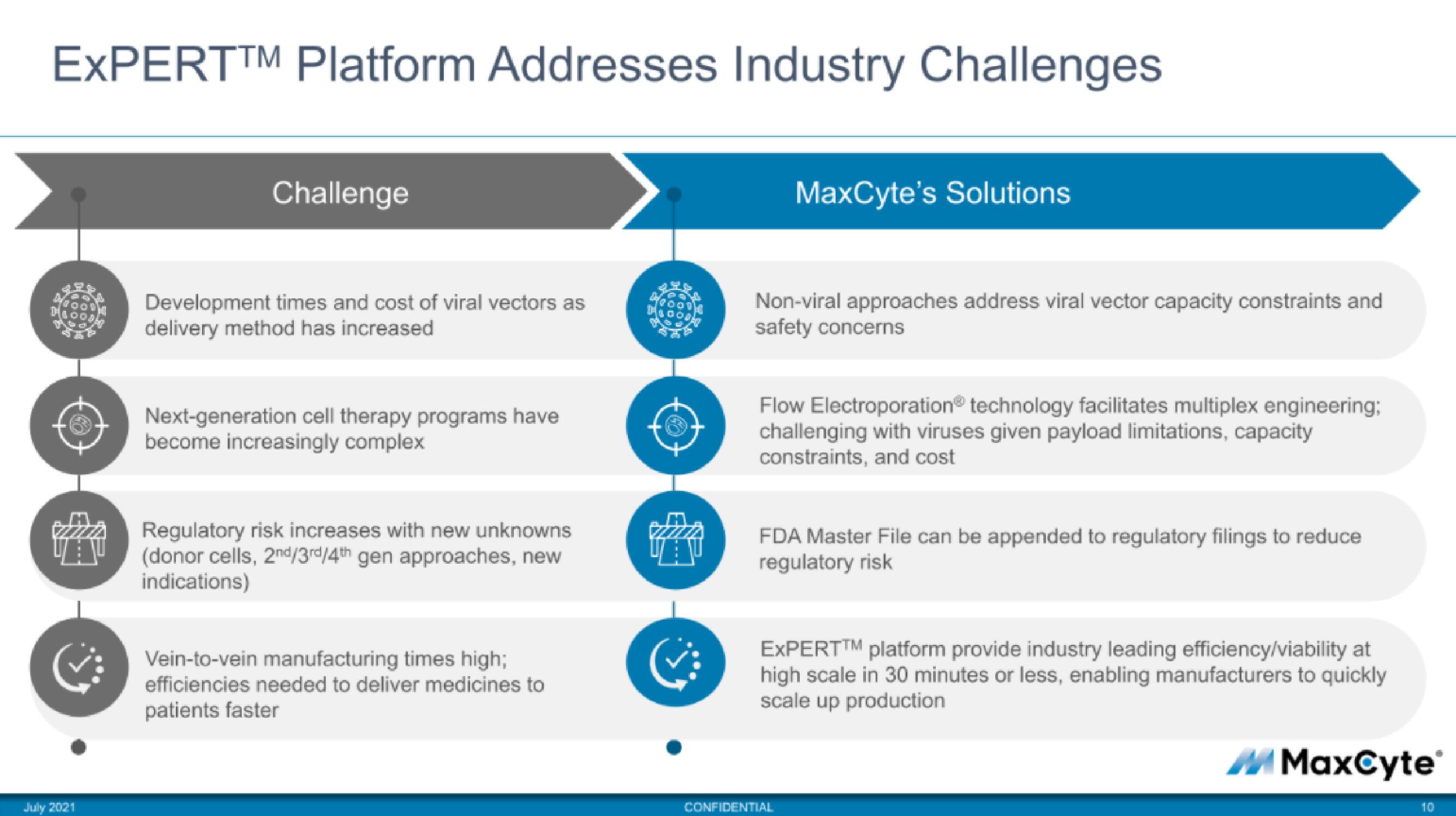
EXPERTTM Platform Addresses Industry Challenges
July 2021
Challenge
Development times and cost of viral vectors as
delivery method has increased
Next-generation cell therapy programs have
become increasingly complex
Regulatory risk increases with new unknowns
(donor cells, 2nd/3rd/4th gen approaches, new
indications)
Vein-to-vein manufacturing times high;
efficiencies needed to deliver medicines to
patients faster
MaxCyte's Solutions
Non-viral approaches address viral vector capacity constraints and
safety concerns
Flow Electroporation technology facilitates multiplex engineering;
challenging with viruses given payload limitations, capacity
constraints, and cost
FDA Master File can be appended to regulatory filings to reduce
regulatory risk
EXPERTTM platform provide industry leading efficiency/viability at
high scale in 30 minutes or less, enabling manufacturers to quickly
scale up production
CONFIDENTIAL
M MaxCyte
10View entire presentation