Investor Presentation
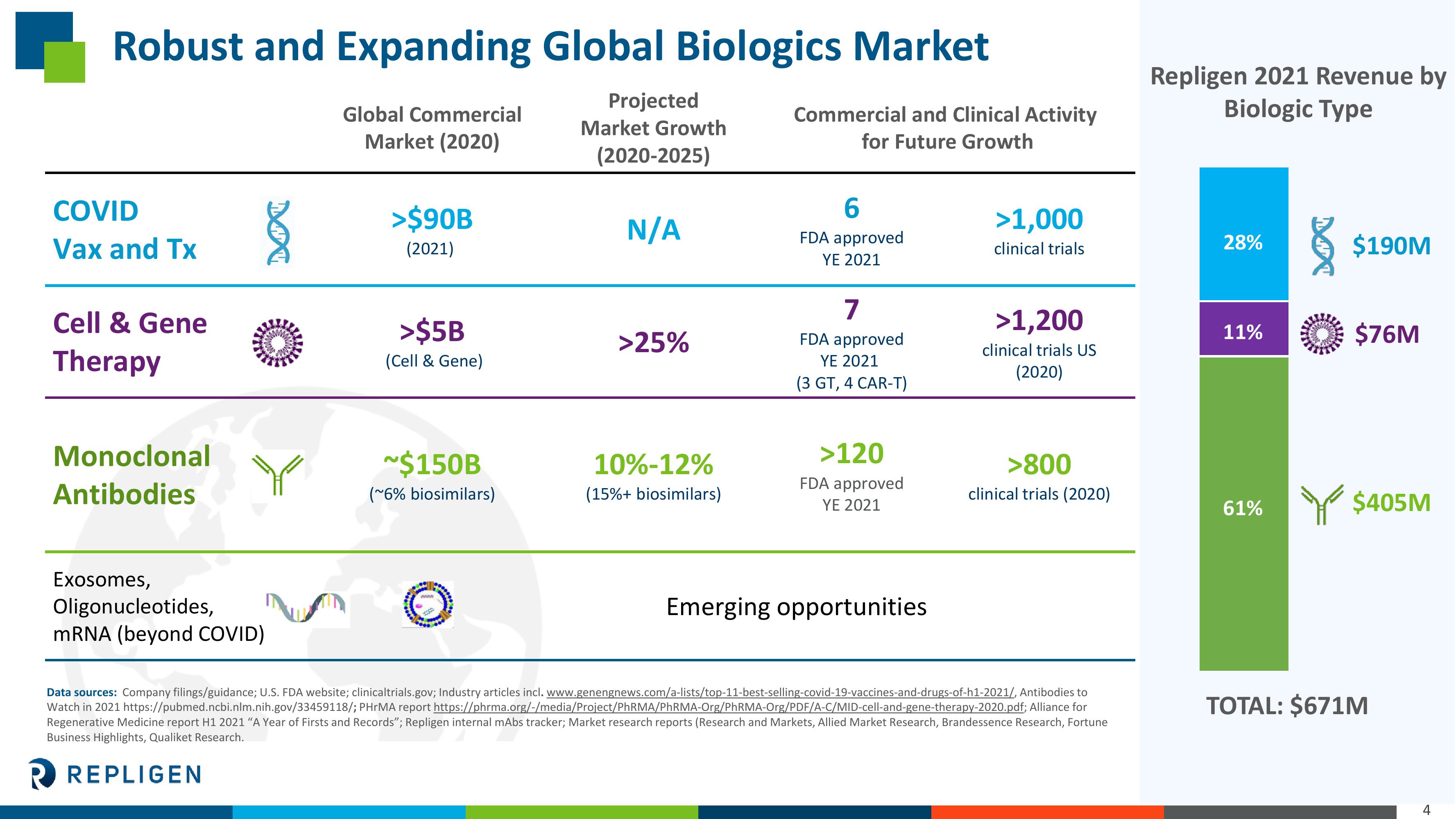
Robust and Expanding Global Biologics Market
COVID
Vax and Tx
Cell & Gene
Therapy
Monoclonal
Antibodies
Exosomes,
Oligonucleotides,
mRNA (beyond COVID)
Global Commercial
Market (2020)
>$90B
(2021)
>$5B
(Cell & Gene)
~$150B
(~6% biosimilars)
13
Projected
Market Growth
(2020-2025)
N/A
>25%
10%-12%
(15%+ biosimilars)
Commercial and Clinical Activity
for Future Growth
6
FDA approved
YE 2021
7
FDA approved
YE 2021
(3 GT, 4 CAR-T)
>120
FDA approved
YE 2021
Emerging opportunities
>1,000
clinical trials
>1,200
clinical trials US
(2020)
>800
clinical trials (2020)
Data sources: Company filings/guidance; U.S. FDA website; clinical trials.gov; Industry articles incl. www.genengnews.com/a-lists/top-11-best-selling-covid-19-vaccines-and-drugs-of-h1-2021/, Antibodies to
Watch in 2021 https://pubmed.ncbi.nlm.nih.gov/33459118/; PHrMA report https://phrma.org/-/media/Project/PhRMA/PhRMA-Org/PhRMA-Org/PDF/A-C/MID-cell-and-gene-therapy-2020.pdf; Alliance for
Regenerative Medicine report H1 2021 "A Year of Firsts and Records"; Repligen internal mAbs tracker; Market research reports (Research and Markets, Allied Market Research, Brandessence Research, Fortune
Business Highlights, Qualiket Research.
R REPLIGEN
Repligen 2021 Revenue by
Biologic Type
28%
11%
61%
$190M
$76M
$405M
TOTAL: $671M
4View entire presentation