AstraZeneca Results Presentation Deck
Best change from baseline (%)
PD-L1 NE
0
-10
-20
-30
-40
-50
-60
-70
-80
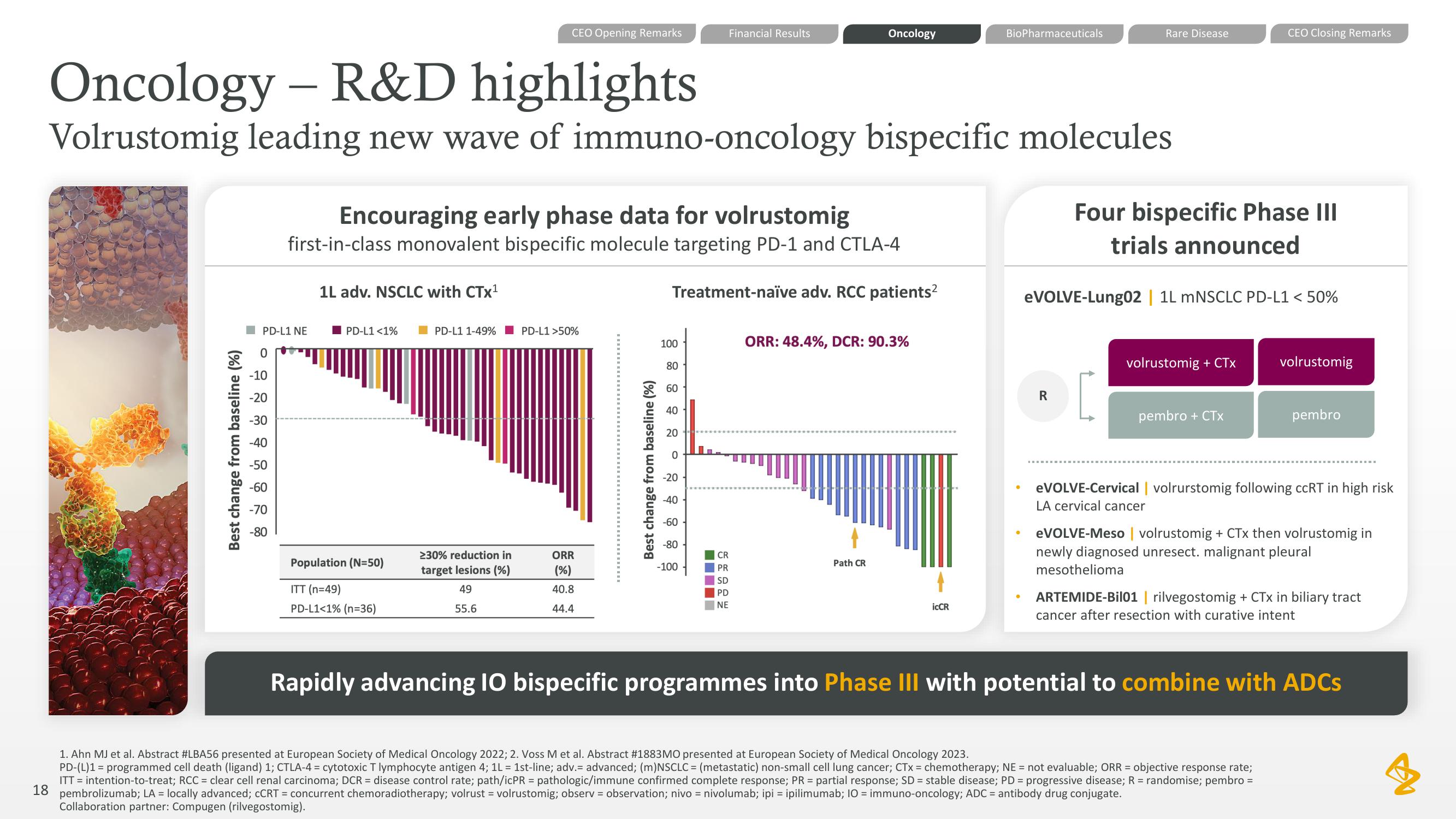
Oncology - R&D highlights
Volrustomig leading new wave of immuno-oncology bispecific molecules
1L adv. NSCLC with CTX¹
Encouraging early phase data for volrustomig
first-in-class monovalent bispecific molecule targeting PD-1 and CTLA-4
Treatment-naïve adv. RCC patients²
PD-L1 <1%
CEO Opening Remarks
Population (N=50)
ITT (n=49)
PD-L1<1% (n=36)
PD-L1 1-49% PD-L1 >50%
>30% reduction in
target lesions (%)
49
55.6
ORR
(%)
40.8
44.4
Best change from baseline (%)
100
80
60
40
20
0
-40
-60
-80
-100
Financial Results
‒‒‒‒‒
CR
PR
SD
PD
NE
Oncology
ORR: 48.4%, DCR: 90.3%
Path CR
BioPharmaceuticals
t
iCCR
Rare Disease
R
Four bispecific Phase III
trials announced
eVOLVE-Lung02 | 1L mNSCLC PD-L1 < 50%
volrustomig + CTx
pembro + CTX
CEO Closing Remarks
volrustomig
eVOLVE-Cervical | volrurstomig following ccRT in high risk
LA cervical cancer
pembro
eVOLVE-Meso | volrustomig + CTx then volrustomig in
newly diagnosed unresect. malignant pleural
mesothelioma
1. Ahn MJ et al. Abstract #LBA56 presented at European Society of Medical Oncology 2022; 2. Voss M et al. Abstract #1883MO presented at European Society of Medical Oncology 2023.
PD-(L)1 = programmed cell death (ligand) 1; CTLA-4 = cytotoxic T lymphocyte antigen 4; 1L = 1st-line; adv.= advanced; (m)NSCLC = (metastatic) non-small cell lung cancer; CTx = chemotherapy; NE = not evaluable; ORR = objective response rate;
ARTEMIDE-Bil01 | rilvegostomig + CTx in biliary tract
cancer after resection with curative intent
ITT = intention-to-treat; RCC = clear cell renal carcinoma; DCR = disease control rate; path/icPR = pathologic/immune confirmed complete response; PR = partial response; SD = stable disease; PD = progressive disease; R = randomise; pembro =
18 pembrolizumab; LA = locally advanced; CCRT = concurrent chemoradiotherapy; volrust = volrustomig; observ = observation; nivo = nivolumab; ipi = ipilimumab; 10 = immuno-oncology; ADC = antibody drug conjugate.
Collaboration partner: Compugen (rilvegostomig).
Rapidly advancing 10 bispecific programmes into Phase III with potential to combine with ADCsView entire presentation