AstraZeneca Investor Day Presentation Deck
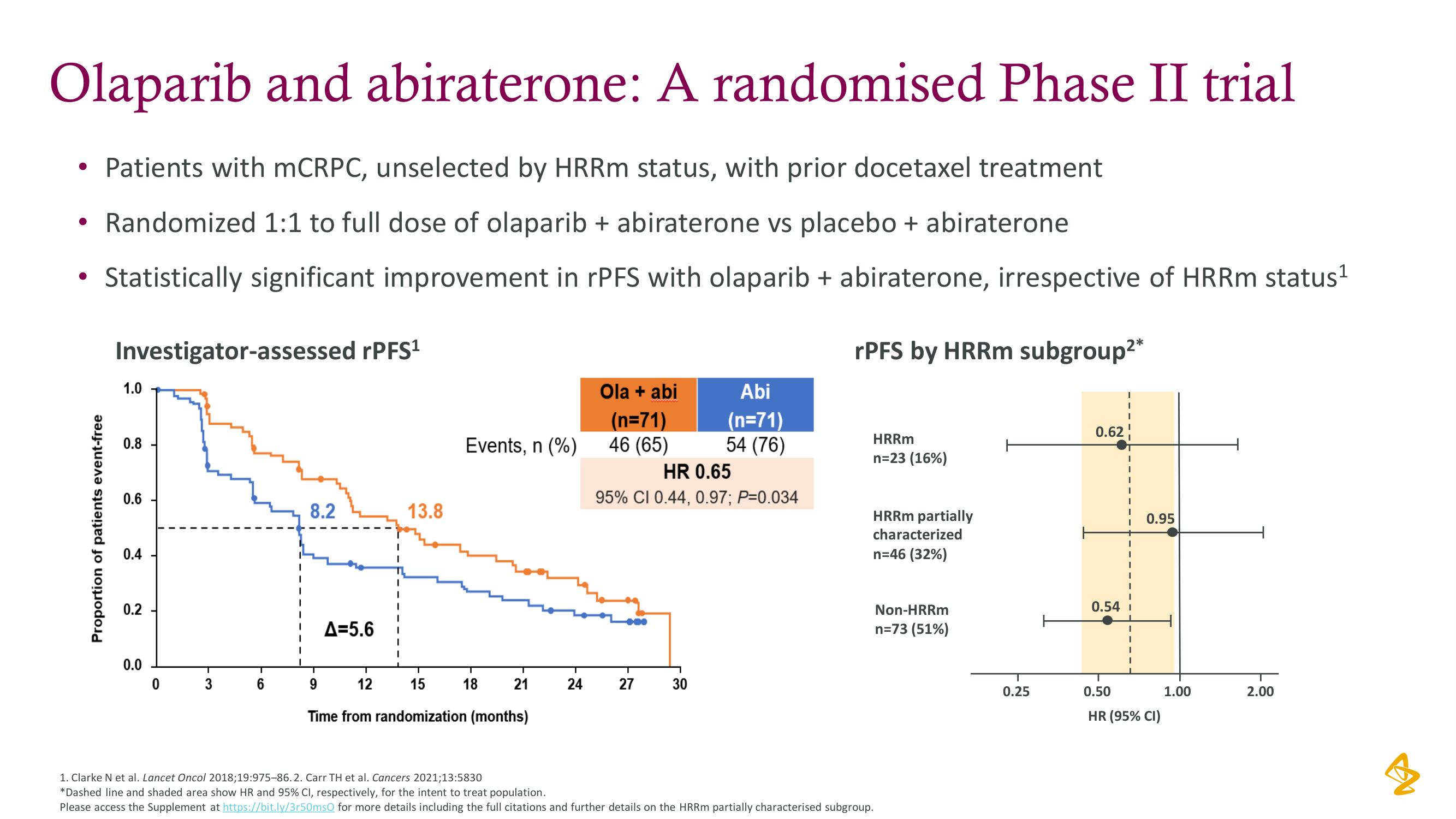
Olaparib and abiraterone: A randomised Phase II trial
Patients with mCRPC, unselected by HRRm status, with prior docetaxel treatment
• Randomized 1:1 to full dose of olaparib + abiraterone vs placebo + abiraterone
●
Statistically significant improvement in rPFS with olaparib + abiraterone, irrespective of HRRm status ¹
rPFS by HRRm subgroup²*
Proportion of patients event-free
Investigator-assessed rPFS¹
1.0
0.8
0.6
0.4
0.2
0.0
0
3
6
8.2
A=5.6
I
I
13.8
Events, n (%)
18
9
12
15
Time from randomization (months)
21
24
Ola + abi
(n=71)
46 (65)
HR 0.65
95% CI 0.44, 0.97; P=0.034
27
Abi
(n=71)
54 (76)
30
HRRm
n=23 (16%)
HRRm partially
characterized
n=46 (32%)
1. Clarke N et al. Lancet Oncol 2018;19:975-86.2. Carr TH et al. Cancers 2021;13:5830
*Dashed line and shaded area show HR and 95% CI, respectively, for the intent to treat population.
Please access the Supplement at https://bit.ly/3r50ms0 for more details including the full citations and further details on the HRRm partially characterised subgroup.
Non-HRRm
n=73 (51%)
0.25
0.62
I
1
I
I
I
I
I
I
0.54 I
I
1
0.95
0.50
HR (95% CI)
1.00
2.00
3View entire presentation