Centessa IPO Presentation Deck
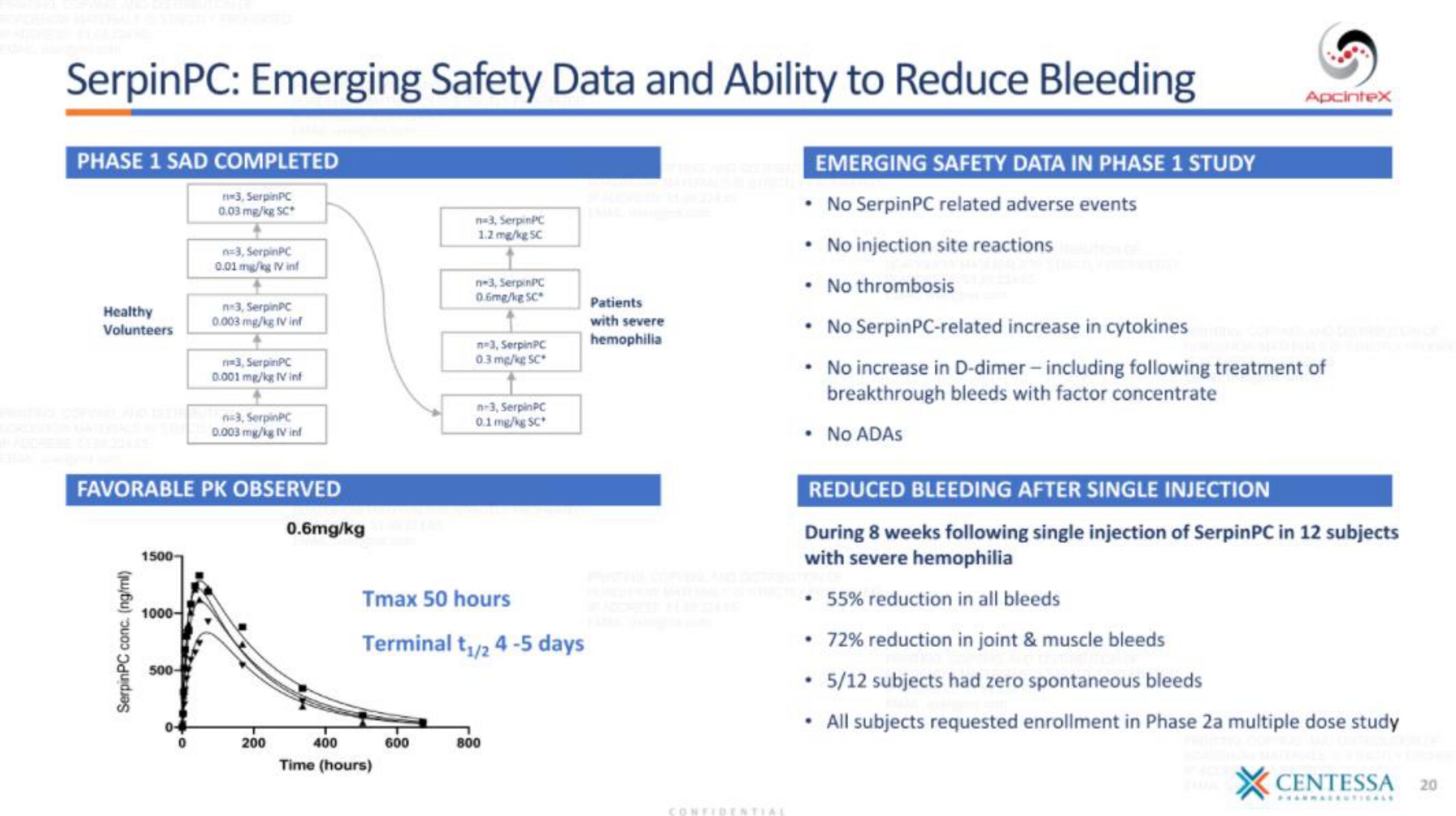
SerpinPC: Emerging Safety Data and Ability to Reduce Bleeding
PHASE 1 SAD COMPLETED
Healthy
Volunteers
SerpinPC conc. (ng/ml)
1500-
n-3, SerpinPC
0.03 mg/kg SC*
SPAVALANK THE n=3, SerpinPC
0.003 mg/kg IV inf
1000-
n=3, SerpinPC
0.01 mg/kg IV inf
FAVORABLE PK OBSERVED
500-
n-3, SerpinPC
0.003 mg/kg IV inf
n=3, SerpinPC
0.001 mg/kg IV inf
200
0.6mg/kg
400
Time (hours)
n=3, SerpinPC
1.2 mg/kg 5C
600
n-3, Serpin PC
0.6mg/kg SC
n-3, SerpinPC
0.3 mg/kg SC*
Tmax 50 hours
Terminal t₁/24 -5 days
n-3, Serpin PC
0.1 mg/kg SC*
800
Patients
with severe
hemophilia
EMERGING SAFETY DATA IN PHASE 1 STUDY
• No SerpinPC related adverse events
• No injection site reactions
• No thrombosis
Apcintex
• No Serpin PC-related increase in cytokines
• No increase in D-dimer - including following treatment of
breakthrough bleeds with factor concentrate
• No ADAs
REDUCED BLEEDING AFTER SINGLE INJECTION
During 8 weeks following single injection of SerpinPC in 12 subjects
with severe hemophilia
• 55% reduction in all bleeds
72% reduction in joint & muscle bleeds
. 5/12 subjects had zero spontaneous bleeds
All subjects requested enrollment in Phase 2a multiple dose study
CENTESSA
20View entire presentation