Ocuphire Pharma Investor Updates
P
14
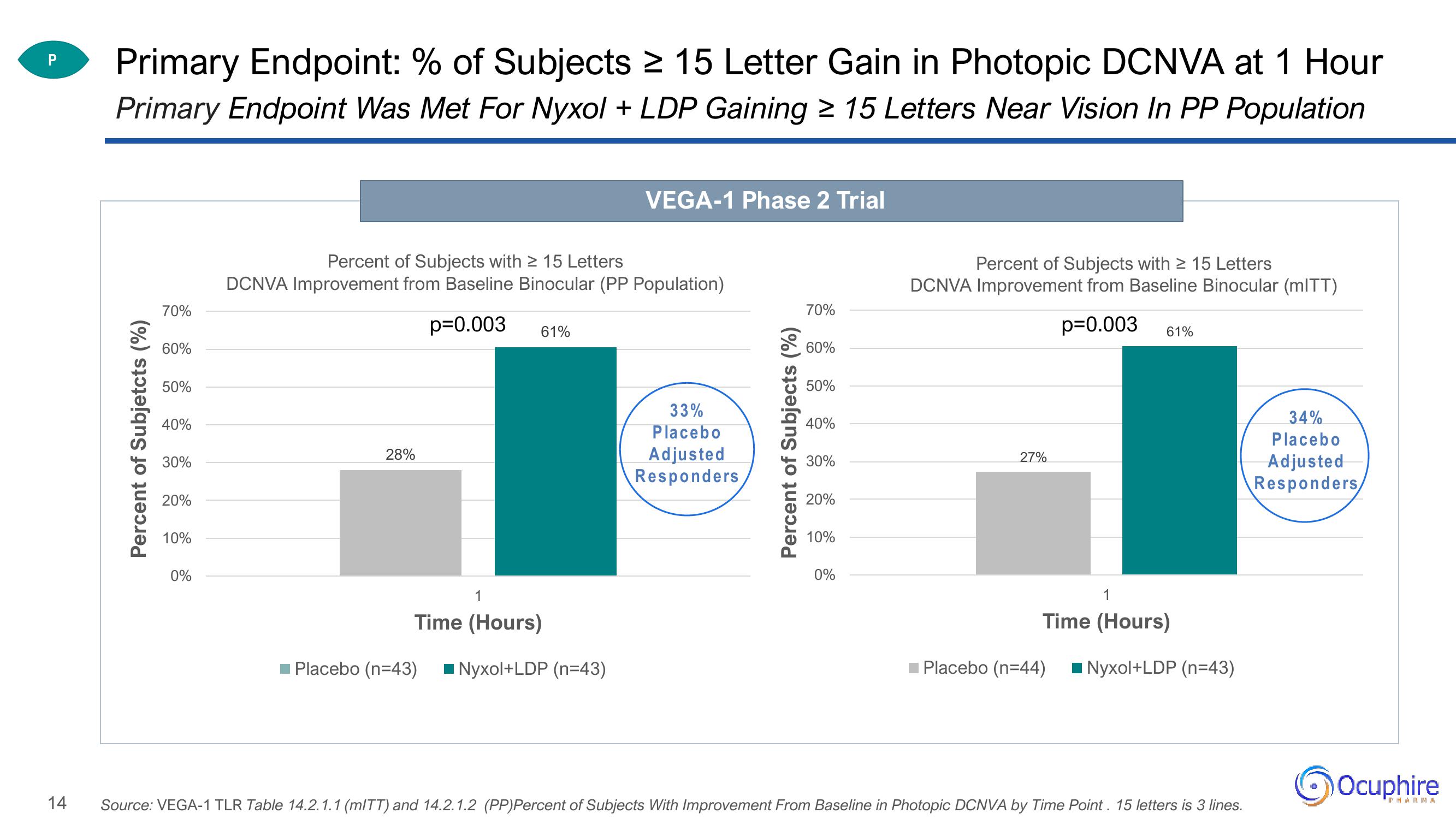
Primary Endpoint: % of Subjects ≥ 15 Letter Gain in Photopic DCNVA at 1 Hour
Primary Endpoint Was Met For Nyxol + LDP Gaining ≥ 15 Letters Near Vision In PP Population
Percent of Subjetcts (%)
70%
60%
50%
40%
30%
20%
10%
0%
Percent of Subjects with ≥ 15 Letters
DCNVA Improvement from Baseline Binocular (PP Population)
p=0.003 61%
28%
1
VEGA-1 Phase 2 Trial
Time (Hours)
Placebo (n=43) Nyxol+LDP (n=43)
33%
Placebo
Adjusted
Responders
Percent of Subjects (%)
70%
60%
50%
40%
30%
20%
10%
0%
Percent of Subjects with ≥ 15 Letters
DCNVA Improvement from Baseline Binocular (mITT)
p=0.003 61%
27%
1
Time (Hours)
Placebo (n=44)
Nyxol+LDP (n=43)
Source: VEGA-1 TLR Table 14.2.1.1 (mITT) and 14.2.1.2 (PP)Percent of Subjects With Improvement From Baseline in Photopic DCNVA by Time Point. 15 letters is 3 lines.
34%
Placebo
Adjusted
Responders,
Ocuphire
PHARMAView entire presentation