BioAtla Investor Presentation Deck
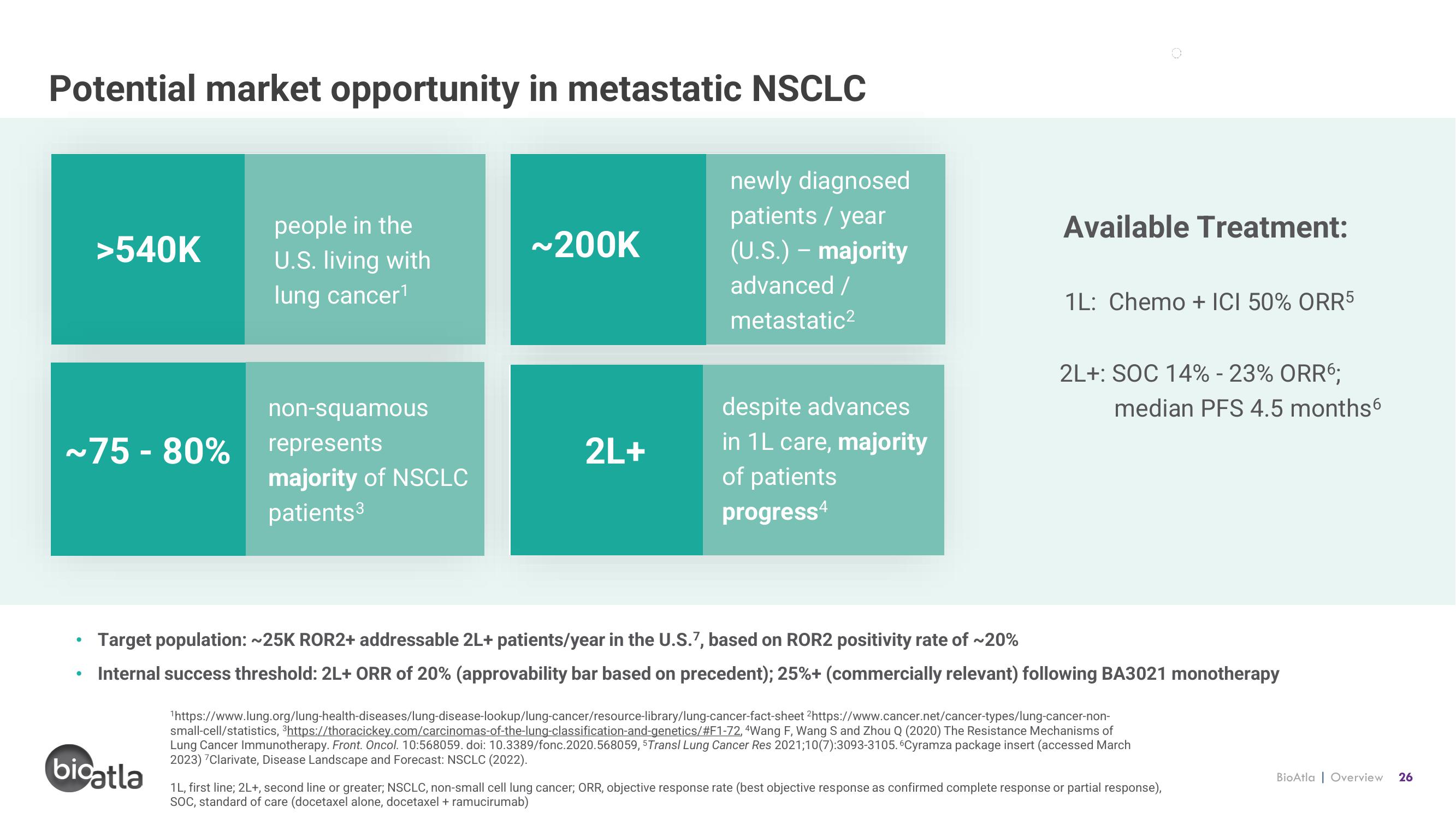
Potential market opportunity in metastatic NSCLC
>540K
~75-80%
●
people in the
U.S. living with
lung cancer¹
bicatla
non-squamous
represents
majority of NSCLC
patients ³
~200K
2L+
newly diagnosed
patients / year
(U.S.) - majority
advanced /
metastatic²
despite advances
in 1L care, majority
of patients
progress4
Available Treatment:
1L: Chemo + ICI 50% ORR5
2L+: SOC 14% -23% ORR6:
median PFS 4.5 months6
Target population: ~25K ROR2+ addressable 2L+ patients/year in the U.S.7, based on ROR2 positivity rate of ~20%
Internal success threshold: 2L+ ORR of 20% (approvability bar based on precedent); 25%+ (commercially relevant) following BA3021 monotherapy
small-cell/statistics,
¹https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet 2https://www.cancer.net/cancer-types/lung-cancer-non-
³https://thoracickey.com/carcinomas-of-the-lung-classification-and-genetics/#F1-72, 4Wang F, Wang S and Zhou Q (2020) The Resistance Mechanisms of
Lung Cancer Immunotherapy. Front. Oncol. 10:568059. doi: 10.3389/fonc.2020.568059, 5Transl Lung Cancer Res 2021;10(7):3093-3105. 6Cyramza package insert (accessed March
2023) 'Clarivate, Disease Landscape and Forecast: NSCLC (2022).
1L, first line; 2L+, second line or greater; NSCLC, non-small cell lung cancer; ORR, objective response rate (best objective response as confirmed complete response or partial response),
SOC, standard of care (docetaxel alone, docetaxel + ramucirumab)
BioAtla| Overview 26View entire presentation