BioAtla Investor Presentation Deck
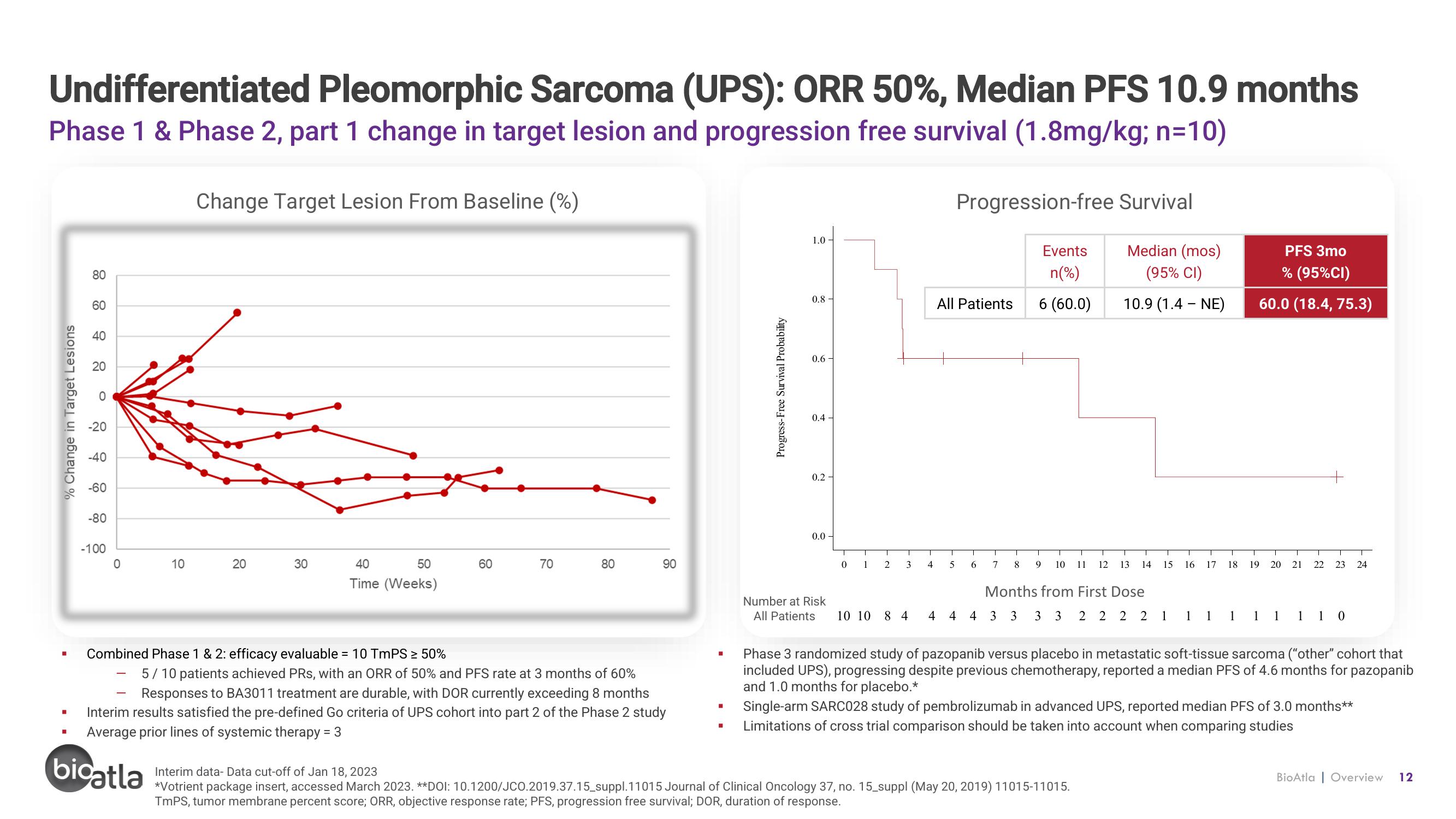
Undifferentiated Pleomorphic Sarcoma (UPS): ORR 50%, Median PFS 10.9 months
Phase 1 & Phase 2, part 1 change in target lesion and progression free survival (1.8mg/kg; n=10)
% Change in Target Lesions
80
■
60
40
20
0
-20
-40
-60
-80
-100
O
10
Change Target Lesion From Baseline (%)
20
30
50
40
Time (Weeks)
Combined Phase 1 & 2: efficacy evaluable = 10 TmPS ≥ 50%
60
70
80
90
5/10 patients achieved PRs, with an ORR of 50% and PFS rate at 3 months of 60%
Responses to BA3011 treatment are durable, with DOR currently exceeding 8 months
Interim results satisfied the pre-defined Go criteria of UPS cohort into part 2 of the Phase 2 study
Average prior lines of systemic therapy = 3
bicatla
■
■
Progress-Free Survival Probability
1.0
0.8
0.6 -
0.4-
0.2
0.0
T
0
1
1
T
T
2 3
Number at Risk
All Patients 10 10 8 4
4
4
Progression-free Survival
All Patients
5
6
T
T
7 8
Events
n(%)
6 (60.0)
Median (mos)
(95% CI)
10.9 (1.4 - NE)
T
T
T
T
9 10 11 12 13 14
15
Months from First Dose
4
4 3 3 3 3 2 2 2 2 1
Interim data- Data cut-off of Jan 18, 2023
*Votrient package insert, accessed March 2023. **DOI: 10.1200/JCO.2019.37.15_suppl. 11015 Journal of Clinical Oncology 37, no. 15_suppl (May 20, 2019) 11015-11015.
TmPS, tumor membrane percent score; ORR, objective response rate; PFS, progression free survival; DOR, duration of response.
16
PFS 3mo
% (95% CI)
60.0 (18.4, 75.3)
T
T
T
T T
17 18 19 20 21 22 23 24
1 1 1 1 1 1 10
Phase 3 randomized study of pazopanib versus placebo in metastatic soft-tissue sarcoma ("other" cohort that
included UPS), progressing despite previous chemotherapy, reported a median PFS of 4.6 months for pazopanib
and 1.0 months for placebo.*
Single-arm SARC028 study of pembrolizumab in advanced UPS, reported median PFS of 3.0 months**
Limitations of cross trial comparison should be taken into account when comparing studies
BioAtla| Overview
12View entire presentation