Immix Biopharma Investor Presentation Deck
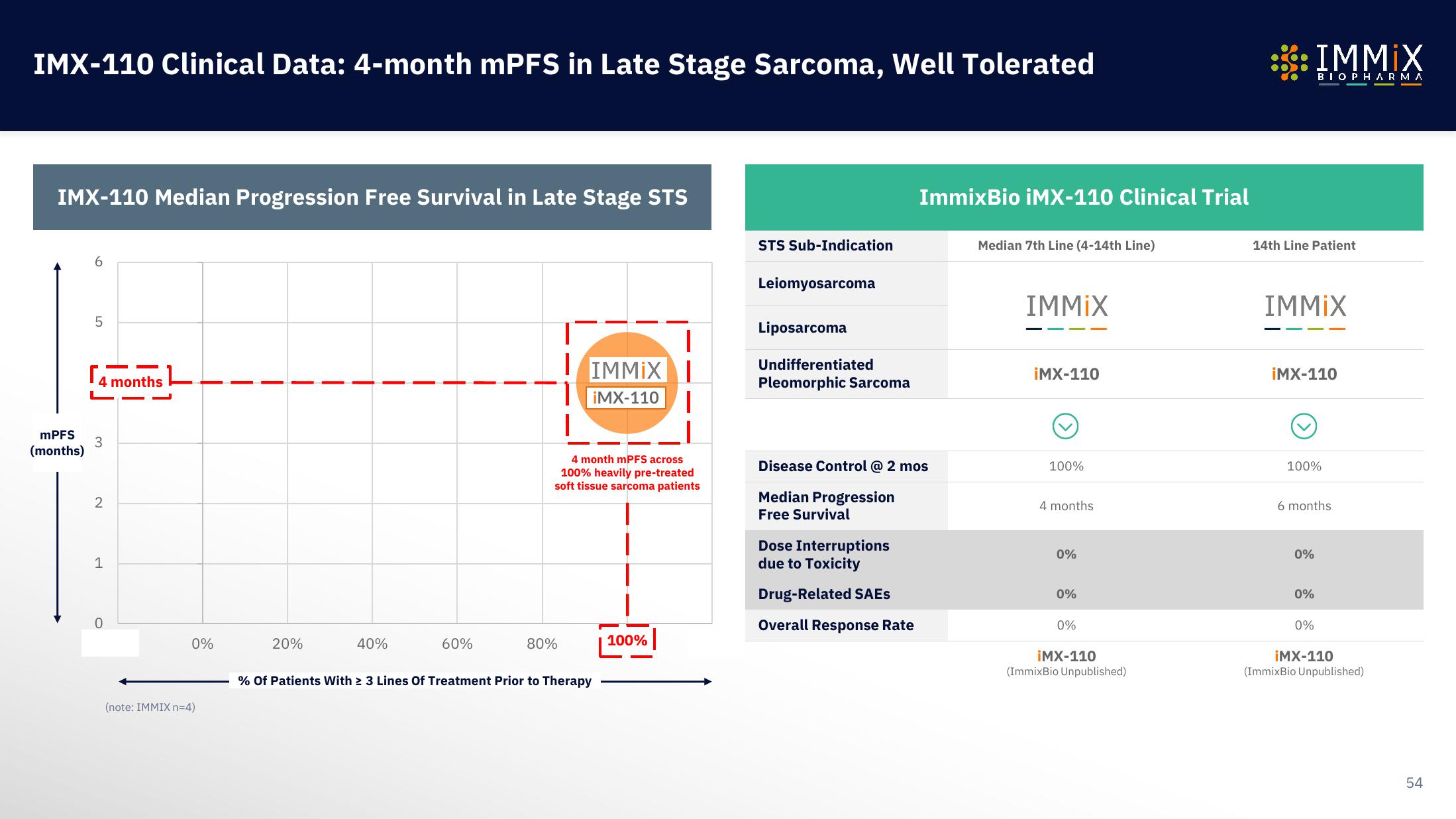
IMX-110 Clinical Data: 4-month mPFS in Late Stage Sarcoma, Well Tolerated
IMX-110 Median Progression Free Survival in Late Stage STS
mPFS
(months)
5
4 months
3
2
1
0
0%
(note: IMMIX n=4)
20%
40%
60%
IMMIX
¡MX-110
4 month mPFS across
100% heavily pre-treated
soft tissue sarcoma patients
80%
% Of Patients With ≥ 3 Lines Of Treatment Prior to Therapy
100%
STS Sub-Indication
Leiomyosarcoma
Liposarcoma
Undifferentiated
Pleomorphic Sarcoma
ImmixBio iMX-110 Clinical Trial
Disease Control @ 2 mos
Median Progression
Free Survival
Dose Interruptions
due to Toxicity
Drug-Related SAEs
Overall Response Rate
Median 7th Line (4-14th Line)
IMMIX
iMX-110
100%
4 months
0%
0%
0%
iMX-110
(ImmixBio Unpublished)
●●●
IMMIX
S BIOPHARMA
14th Line Patient
IMMIX
¡MX-110
100%
6 months
0%
0%
0%
¡MX-110
(ImmixBio Unpublished)
54View entire presentation