Ocuphire Pharma Investor Update
7
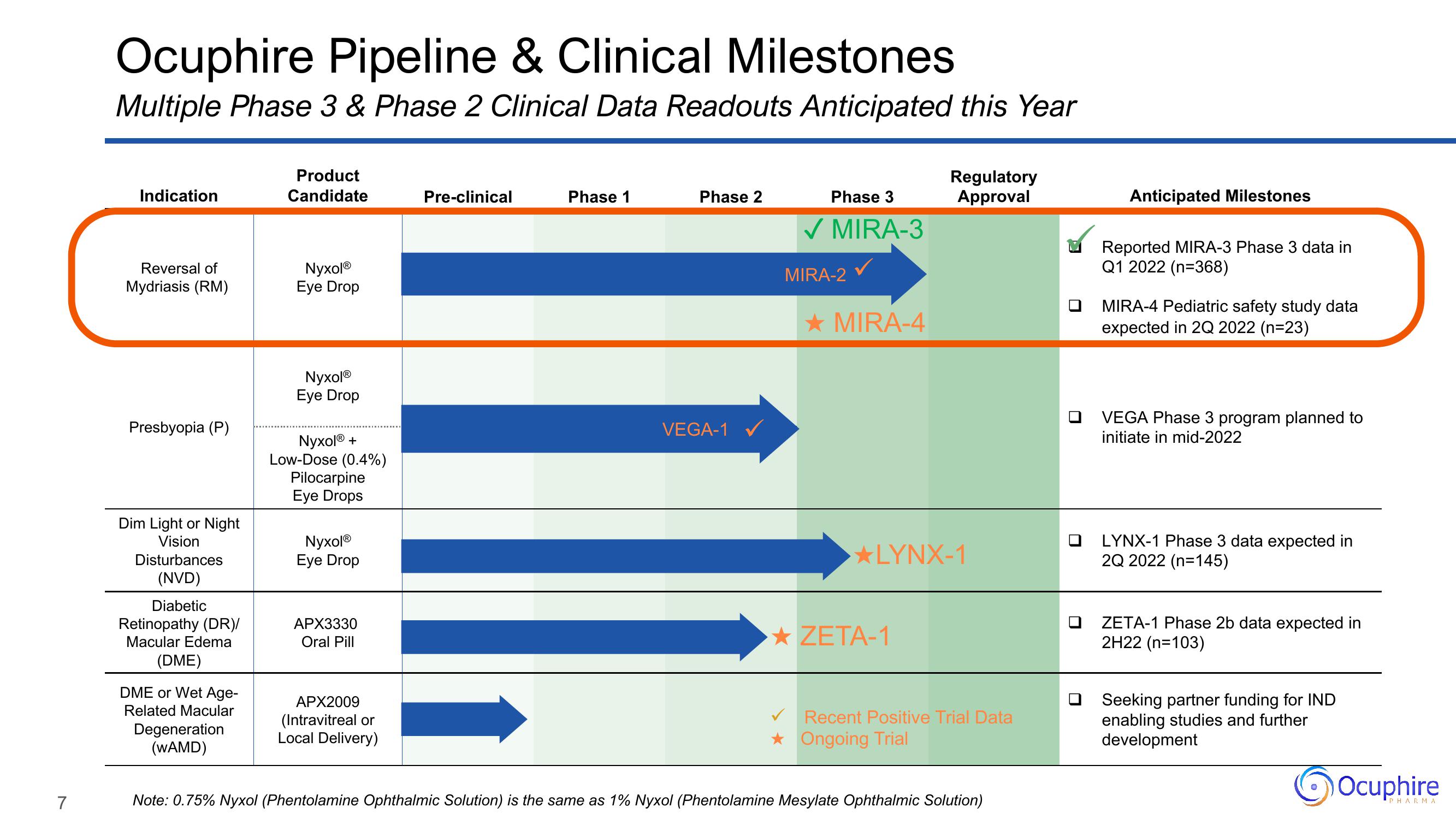
Ocuphire Pipeline & Clinical Milestones
Multiple Phase 3 & Phase 2 Clinical Data Readouts Anticipated this Year
Indication
Reversal of
Mydriasis (RM)
Presbyopia (P)
Dim Light or Night
Vision
Disturbances
(NVD)
Diabetic
Retinopathy (DR)/
Macular Edema
(DME)
DME or Wet Age-
Related Macular
Degeneration
(WAMD)
Product
Candidate
NyxolⓇ
Eye Drop
Nyxol®
Eye Drop
Nyxol® +
Low-Dose (0.4%)
Pilocarpine
Eye Drops
NyxolⓇ
Eye Drop
APX3330
Oral Pill
APX2009
(Intravitreal or
Local Delivery)
Pre-clinical
Phase 1
Phase 2
VEGA-1 ✓
Phase 3
✓ MIRA-3
MIRA-2
★ MIRA-4
Regulatory
Approval
★LYNX-1
★ZETA-1
Recent Positive Trial Data
Ongoing Trial
Note: 0.75% Nyxol (Phentolamine Ophthalmic Solution) is the same as 1% Nyxol (Phentolamine Mesylate Ophthalmic Solution)
Anticipated Milestones
Reported MIRA-3 Phase 3 data in
Q1 2022 (n=368)
MIRA-4 Pediatric safety study data
expected in 2Q 2022 (n=23)
VEGA Phase 3 program planned to
initiate in mid-2022
LYNX-1 Phase 3 data expected in
2Q 2022 (n=145)
ZETA-1 Phase 2b data expected in
2H22 (n=103)
Seeking partner funding for IND
enabling studies and further
development
Ocuphire
PHARMAView entire presentation