Ocuphire Pharma Investor Day Presentation Deck
DR
DME
16
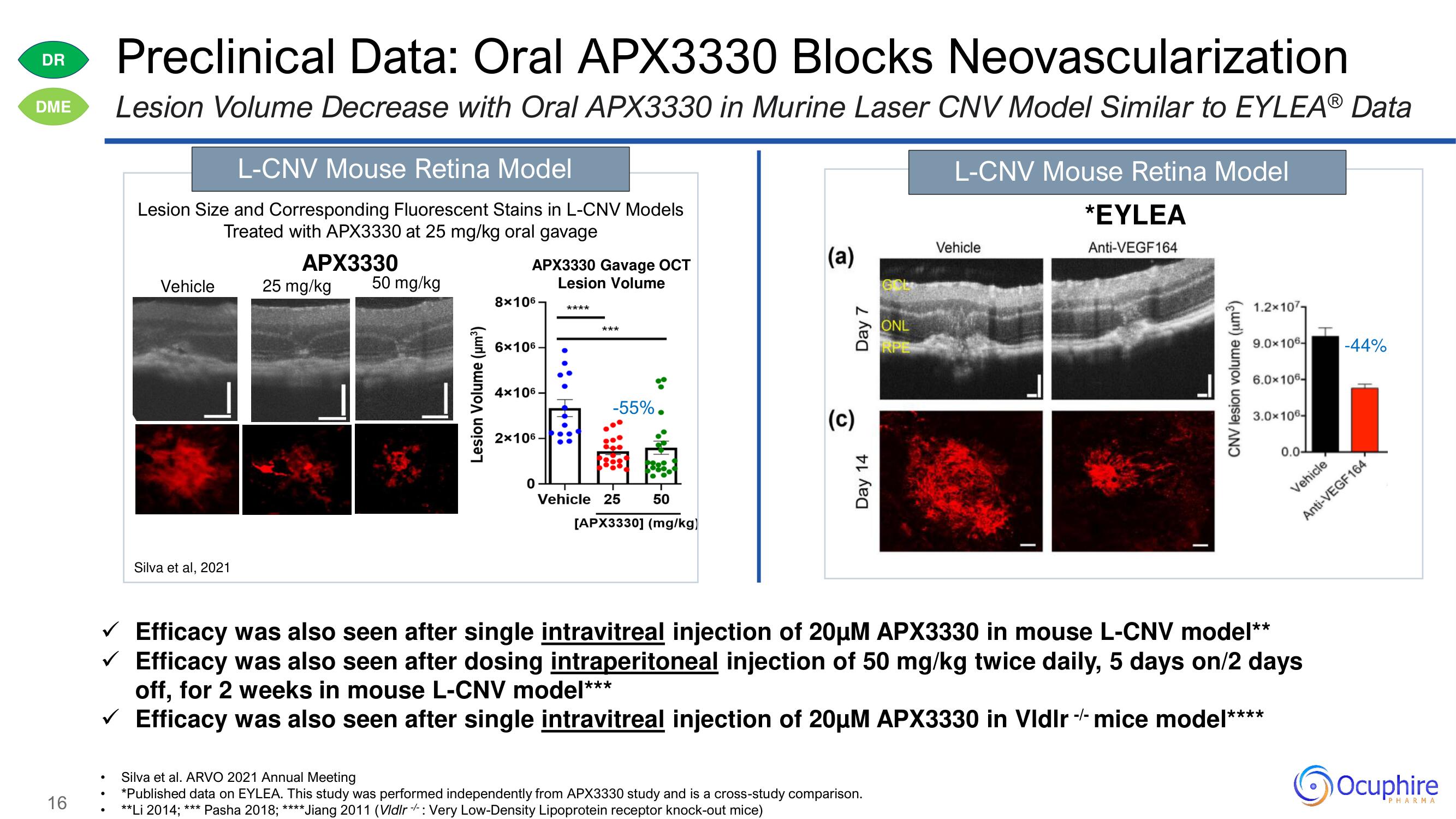
Preclinical Data: Oral APX3330 Blocks Neovascularization
Lesion Volume Decrease with Oral APX3330 in Murine Laser CNV Model Similar to EYLEAⓇ Data
●
L-CNV Mouse Retina Model
Lesion Size and Corresponding Fluorescent Stains in L-CNV Models
Treated with APX3330 at 25 mg/kg oral gavage
APX3330
50 mg/kg
Vehicle
Silva et al, 2021
25 mg/kg
Lesion Volume (µm³)
APX3330 Gavage OCT
Lesion Volume
8x106- ****
6x106
4x106
2x106-
0
***
-55%.
Vehicle 25
50
[APX3330] (mg/kg)
(a)
Day 7
(c)
Day 14
ONL
RPE
Silva et al. ARVO 2021 Annual Meeting
*Published data on EYLEA. This study was performed independently from APX3330 study and is a cross-study comparison.
****
**Li 2014; *** Pasha 2018; *Jiang 2011 (Vldlr : Very Low-Density Lipoprotein receptor knock-out mice)
L-CNV Mouse Retina Model
*EYLEA
Anti-VEGF164
Vehicle
CNV lesion volume (um³)
1.2x107-
9.0x106-
6.0x106-
3.0x106-
0.0
✓ Efficacy was also seen after single intravitreal injection of 20μµM APX3330 in mouse L-CNV model**
✓ Efficacy was also seen after dosing intraperitoneal injection of 50 mg/kg twice daily, 5 days on/2 days
off, for 2 weeks in mouse L-CNV model***
✓
Efficacy was also seen after single intravitreal injection of 20µM APX3330 in Vldlrmice model***
**
Vehicle
-44%
Anti-VEGF164
Ocuphire
PHARMAView entire presentation