Genelux Investor Presentation Deck
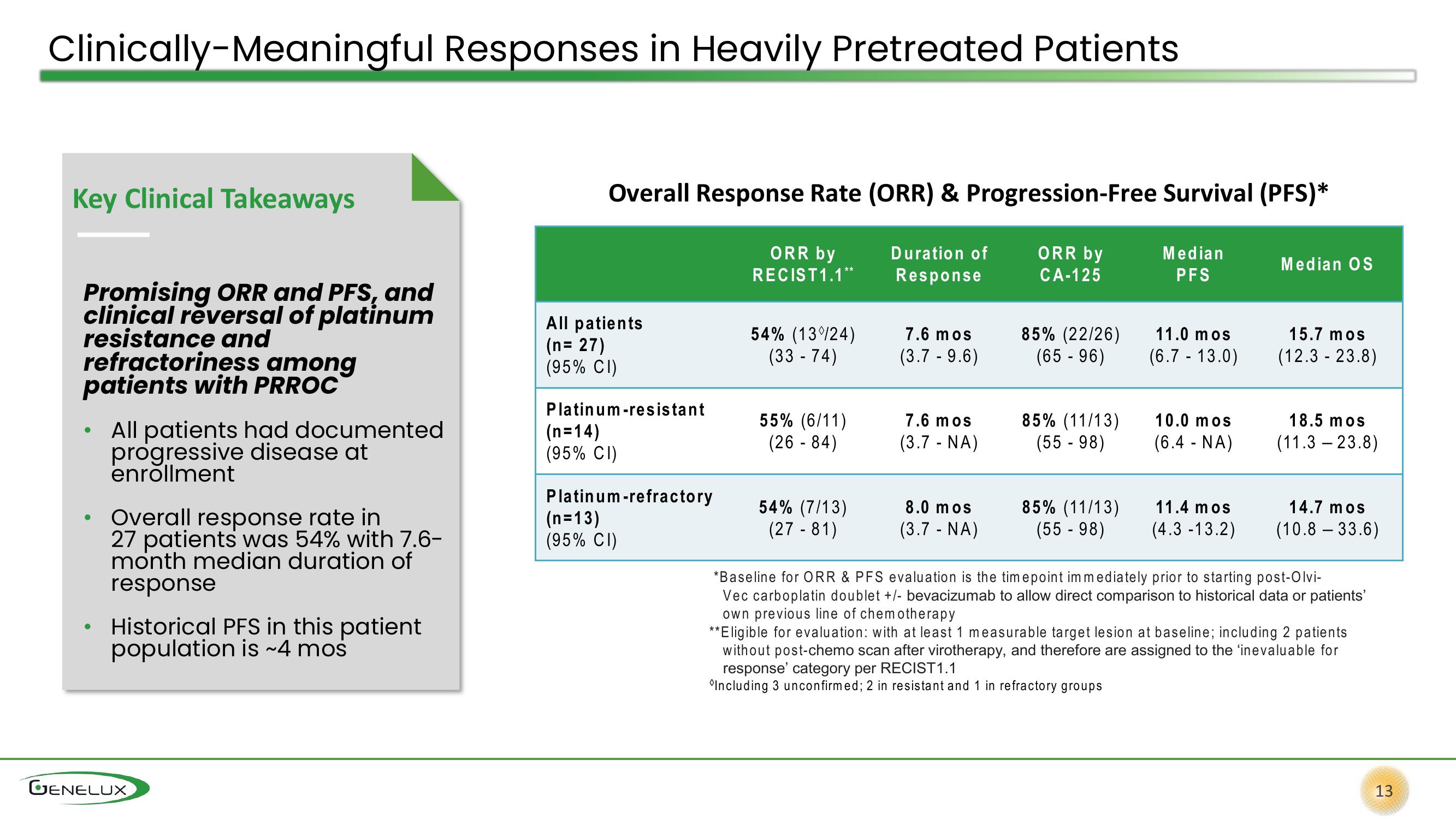
Clinically-Meaningful Responses in Heavily Pretreated Patients
Key Clinical Takeaways
Promising ORR and PFS, and
clinical reversal of platinum
resistance and
refractoriness among
patients with PRROC
●
●
All patients had documented
progressive disease at
enrollment
Overall response rate in
27 patients was 54% with 7.6-
month median duration of
response
Historical PFS in this patient
population is ~4 mos
GENELUX
Overall Response Rate (ORR) & Progression-Free Survival (PFS)*
ORR by
RECIST 1.1**
ORR by
CA-125
Median
PFS
All patients
(n=27)
(95% CI)
Platinum-resistant
(n=14)
(95% CI)
Platinum-refractory
(n=13)
(95% CI)
54% (13%24)
(33-74)
55% (6/11)
(26-84)
54% (7/13)
(27-81)
Duration
Response
7.6 mos
(3.7 - 9.6)
7.6 mos
(3.7 - NA)
8.0 mos
(3.7 - NA)
85% (22/26)
(65 - 96)
85% (11/13)
(55-98)
85% (11/13)
(55-98)
11.0 mos
(6.7 - 13.0)
10.0 mos
(6.4 - NA)
11.4 mos
(4.3 -13.2)
Median OS
15.7 mos
(12.3 - 23.8)
18.5 mos
(11.323.8)
14.7 mos
(10.8 -33.6)
*Baseline for ORR & PFS evaluation is the timepoint immediately prior to starting post-Olvi-
Vec carboplatin doublet +/- bevacizumab to allow direct comparison to historical data or patients'
own previous line of chemotherapy
**Eligible for evaluation: with at least 1 measurable target lesion at baseline; including 2 patients
without post-chemo scan after virotherapy, and therefore are assigned to the 'inevaluable for
response' category per RECIST1.1
"Including 3 unconfirmed; 2 in resistant and 1 in refractory groups
13View entire presentation