BioAtla Investor Presentation Deck
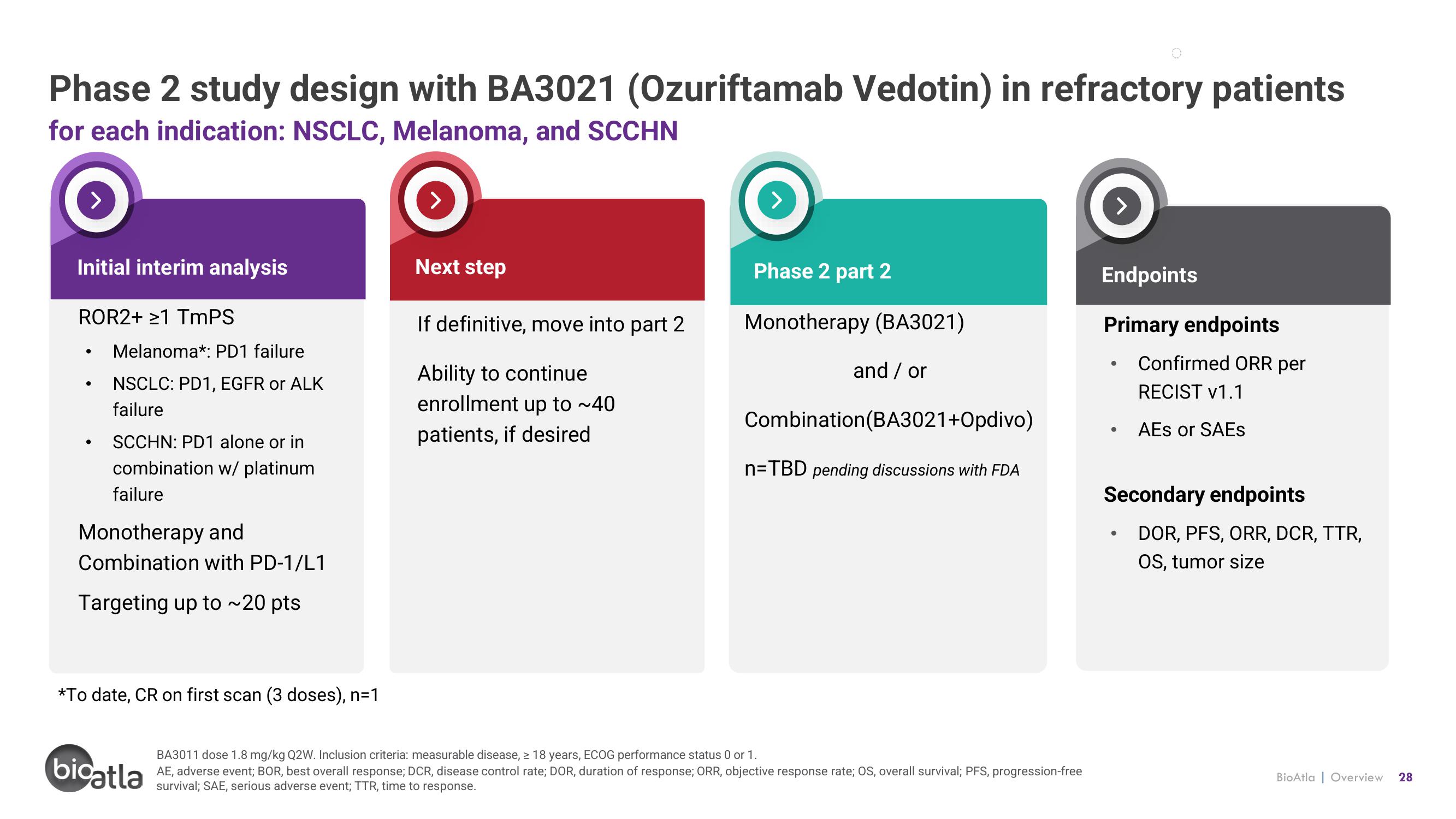
Phase 2 study design with BA3021 (Ozuriftamab Vedotin) in refractory patients
for each indication: NSCLC, Melanoma, and SCCHN
Initial interim analysis
ROR2+ ≥1 TmPS
●
●
Melanoma*: PD1 failure
NSCLC: PD1, EGFR or ALK
failure
SCCHN: PD1 alone or in
combination w/ platinum
failure
Monotherapy and
Combination with PD-1/L1
Targeting up to ~20 pts
*To date, CR on first scan (3 doses), n=1
Next step
If definitive, move into part 2
Ability to continue
enrollment up to ~40
patients, if desired
Phase 2 part 2
Monotherapy (BA3021)
and / or
Combination (BA3021+Opdivo)
n=TBD pending discussions with FDA
BA3011 dose 1.8 mg/kg Q2W. Inclusion criteria: measurable disease, ≥ 18 years, ECOG performance status 0 or 1.
bicatla AE, adverse event, BOR, best overall response; DCR, disease control rate; DOR, duration of response; ORR, objective response rate; OS, overall survival; PFS, progression-free
survival; SAE, serious adverse event; TTR, time to response.
Endpoints
Primary endpoints
●
●
Confirmed ORR per
RECIST v1.1
AES or SAEs
Secondary endpoints
●
DOR, PFS, ORR, DCR, TTR,
OS, tumor size
BioAtla| Overview 28View entire presentation