AstraZeneca Results Presentation Deck
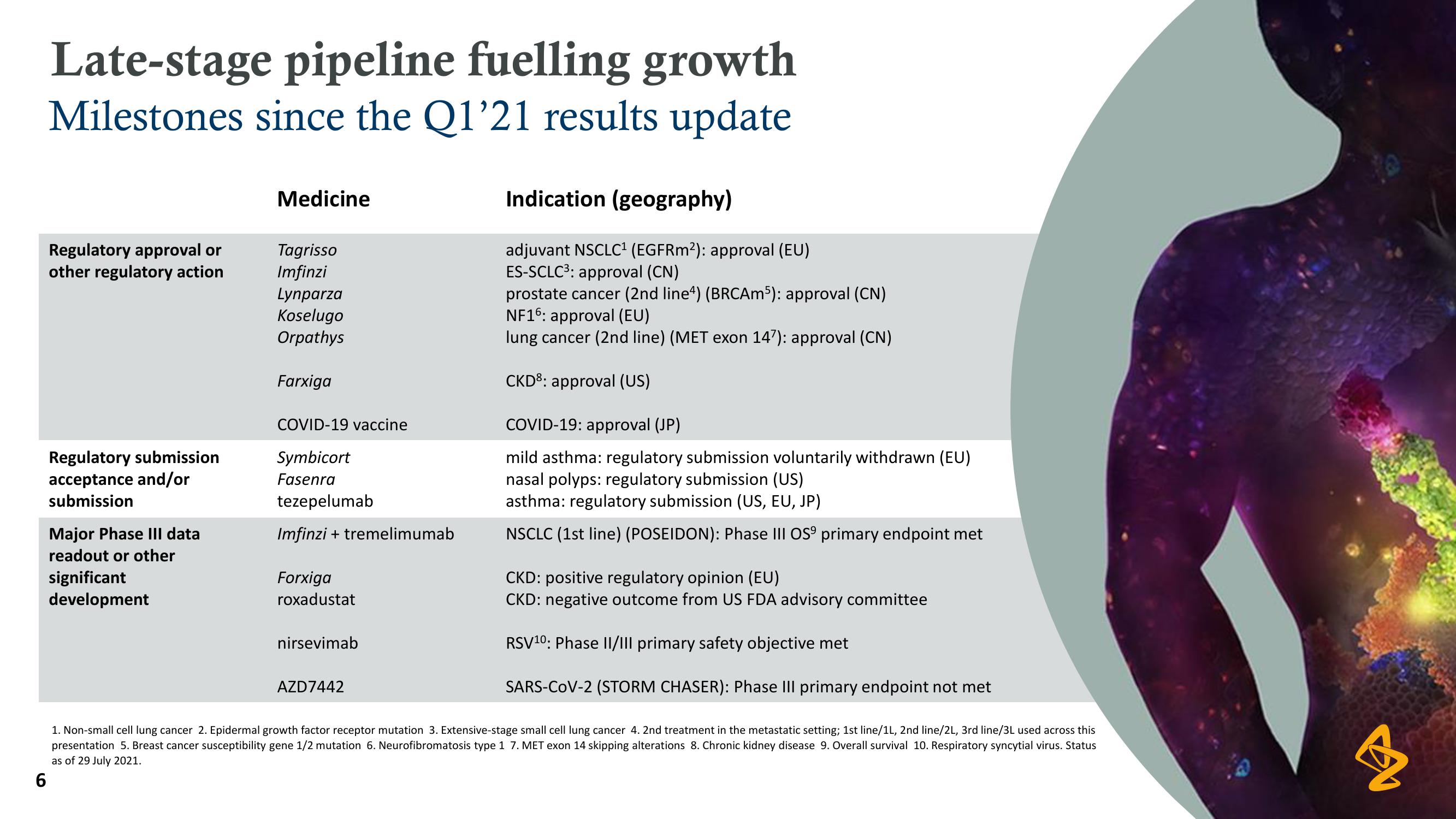
Late-stage pipeline fuelling growth
Milestones since the Q1'21 results update
Regulatory approval or
other regulatory action
Regulatory submission
acceptance and/or
submission
Major Phase III data
readout or other
significant
development
Medicine
Tagrisso
Imfinzi
Lynparza
Koselugo
Orpathys
Farxiga
COVID-19 vaccine
Symbicort
Fasenra
tezepelumab
Imfinzi + tremelimumab
Forxiga
roxadustat
nirsevimab
AZD7442
Indication (geography)
adjuvant NSCLC1¹ (EGFRm2): approval (EU)
ES-SCLC³: approval (CN)
prostate cancer (2nd line4) (BRCAm5): approval (CN)
NF16: approval (EU)
lung cancer (2nd line) (MET exon 147): approval (CN)
CKD8: approval (US)
COVID-19: approval (JP)
mild asthma: regulatory submission voluntarily withdrawn (EU)
nasal polyps: regulatory submission (US)
asthma: regulatory submission (US, EU, JP)
NSCLC (1st line) (POSEIDON): Phase III OS9 primary endpoint met
CKD: positive regulatory opinion (EU)
CKD: negative outcome from US FDA advisory committee
RSV10: Phase II/III primary safety objective met
SARS-CoV-2 (STORM CHASER): Phase III primary endpoint not met
1. Non-small cell lung cancer 2. Epidermal growth factor receptor mutation 3. Extensive-stage small cell lung cancer 4. 2nd treatment in the metastatic setting; 1st line/1L, 2nd line/2L, 3rd line/3L used across this
presentation 5. Breast cancer susceptibility gene 1/2 mutation 6. Neurofibromatosis type 1 7. MET exon 14 skipping alterations 8. Chronic kidney disease 9. Overall survival 10. Respiratory syncytial virus. Status
as of 29 July 2021.
6
4View entire presentation