Immix Biopharma Investor Presentation Deck
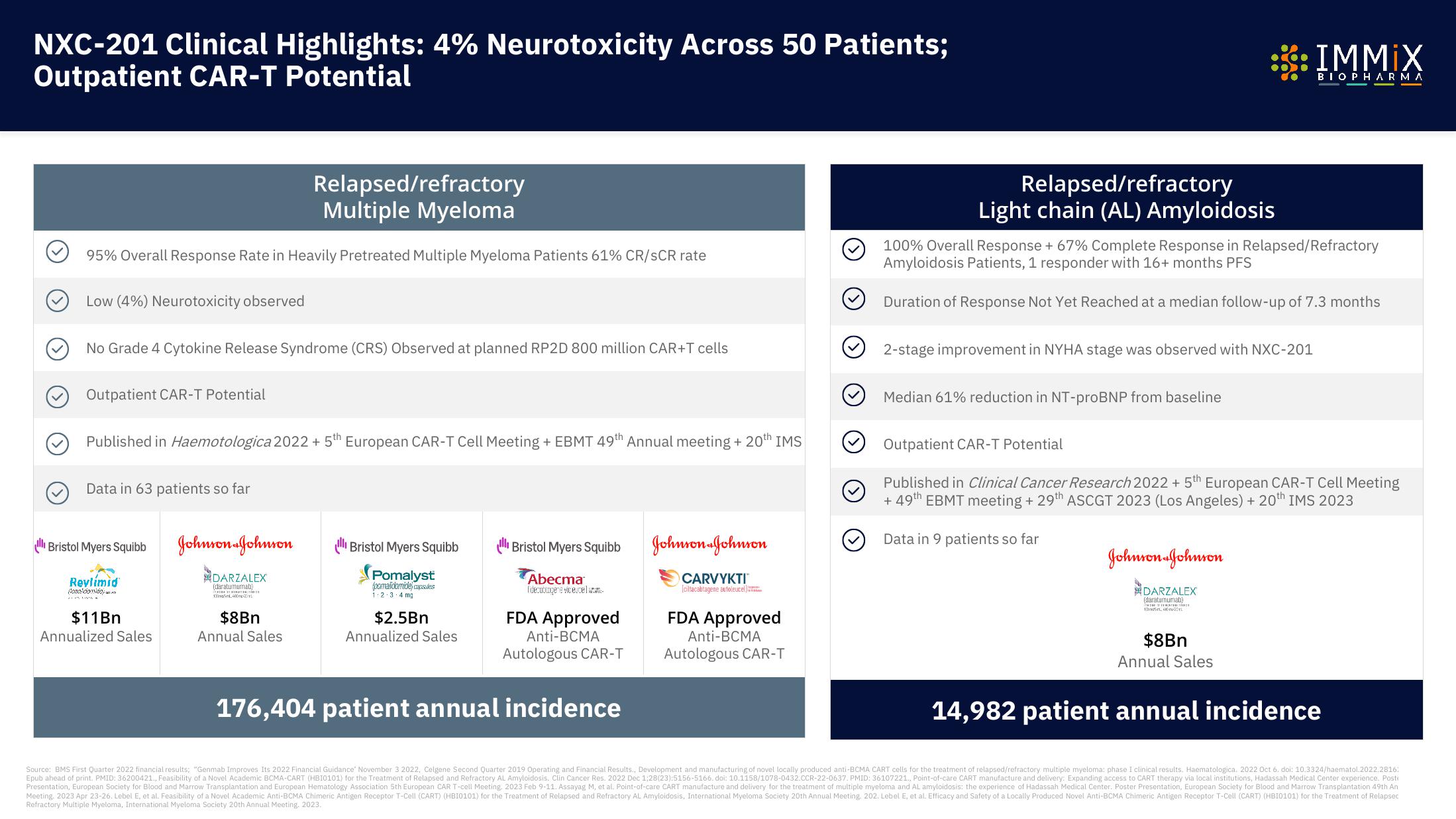
NXC-201 Clinical Highlights: 4% Neurotoxicity Across 50 Patients;
Outpatient CAR-T Potential
Relapsed/refractory
Multiple Myeloma
95% Overall Response Rate in Heavily Pretreated Multiple Myeloma Patients 61% CR/SCR rate
Low (4%) Neurotoxicity observed
No Grade 4 Cytokine Release Syndrome (CRS) Observed at planned RP2D 800 million CAR+T cells
Outpatient CAR-T Potential
Published in Haemotologica 2022 + 5th European CAR-T Cell Meeting + EBMT 49th Annual meeting + 20th IMS
Data in 63 patients so far
ll Bristol Myers Squibb
Revlimid
Ronaldomidoves
$11Bn
Annualized Sales
Johnson & Johnson
DARZALEX
(daratumumab)
A
Ill Bristol Myers Squibb
Pomalyst
foomalioomide) capsules
1-2-3-4 mg
$2.5Bn
Annualized Sales
$8Bn
Annual Sales
Ill Bristol Myers Squibb
Abecma
fidecubungene viceucel
FDA Approved
Anti-BCMA
Autologous CAR-T
176,404 patient annual incidence
Johnson & Johnson
CARVYKTI™
[ciltacabtagene autoleucel)
FDA Approved
Anti-BCMA
Autologous CAR-T
Relapsed/refractory
Light chain (AL) Amyloidosis
100% Overall Response + 67% Complete Response in Relapsed/Refractory
Amyloidosis Patients, 1 responder with 16+ months PFS
Duration of Response Not Yet Reached at a median follow-up of 7.3 months
2-stage improvement in NYHA stage was observed with NXC-201
Median 61% reduction in NT-proBNP from baseline
●●●
IMMİX
S BIOPHARMA
Outpatient CAR-T Potential
Published in Clinical Cancer Research 2022 + 5th European CAR-T Cell Meeting
+ 49th EBMT meeting + 29th ASCGT 2023 (Los Angeles) + 20th IMS 2023
Data in 9 patients so far
Johnson-Johnson
DARZALEX
(daratumumab)
10
$8Bn
Annual Sales
14,982 patient annual incidence
Source: BMS First Quarter 2022 financial results; "Genmab Improves Its 2022 Financial Guidance' November 3 2022, Celgene Second Quarter 2019 Operating and Financial Results., Development and manufacturing of novel locally produced anti-BCMA CART cells for the treatment of relapsed/refractory multiple myeloma: phase I clinical results. Haematologica. 2022 Oct 6. doi: 10.3324/haematol.2022.2816:
Epub ahead of print. PMID: 36200421., Feasibility of a Novel Academic BCMA-CART (HB10101) for the Treatment of Relapsed and Refractory AL Amyloidosis. Clin Cancer Res. 2022 Dec 1;28(23):5156-5166. doi: 10.1158/1078-0432.CCR-22-0637. PMID: 36107221., Point-of-care CART manufacture and delivery: Expanding access to CART therapy via local institutions, Hadassah Medical Center experience. Posti
Presentation, European Society for Blood and Marrow Transplantation and European Hematology Association 5th European CAR T-cell Meeting. 2023 Feb 9-11. Assayag M, et al. Point-of-care CART manufacture and delivery for the treatment of multiple myeloma and AL amyloidosis: the experience of Hadassah Medical Center. Poster Presentation, European Society for Blood and Marrow Transplantation 49th An
Meeting. 2023 Apr 23-26. Lebel E, et al. Feasibility of a Novel Academic Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HB10101) for the Treatment of Relapsed and Refractory AL Amyloidosis, International Myeloma Society 20th Annual Meeting 202. Lebel E, et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsec
Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting. 2023.View entire presentation