Nuvectis Pharma Investor Presentation Deck
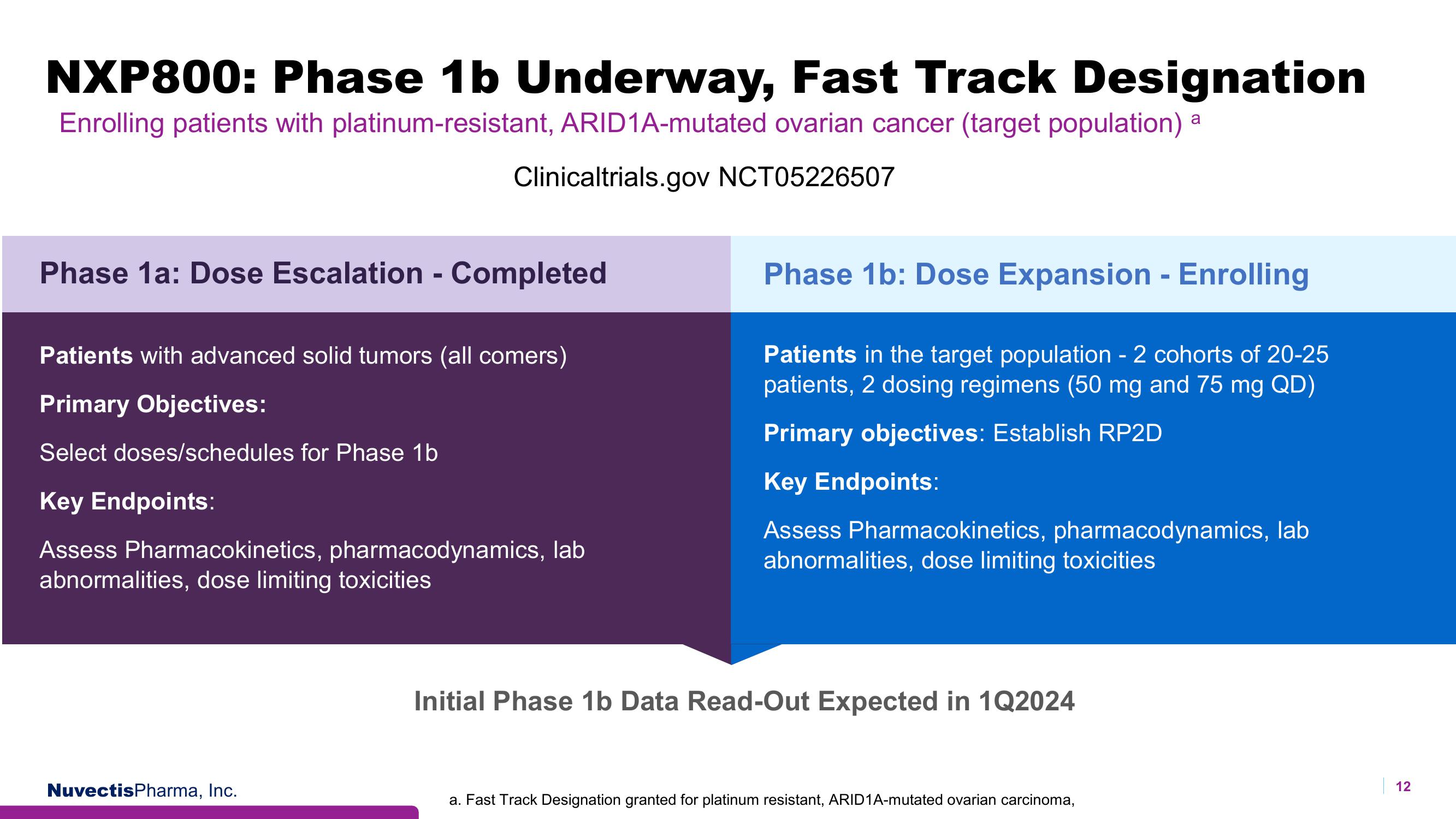
NXP800: Phase 1b Underway, Fast Track Designation
Enrolling patients with platinum-resistant, ARID1A-mutated ovarian cancer (target population) a
Clinicaltrials.gov NCT05226507
Phase 1a: Dose Escalation - Completed
Patients with advanced solid tumors (all comers)
Primary Objectives:
Select doses/schedules for Phase 1b
Key Endpoints:
Assess Pharmacokinetics, pharmacodynamics, lab
abnormalities, dose limiting toxicities
NuvectisPharma, Inc.
Phase 1b: Dose Expansion - Enrolling
Patients in the target population - 2 cohorts of 20-25
patients, 2 dosing regimens (50 mg and 75 mg QD)
Primary objectives: Establish RP2D
Key Endpoints:
Assess Pharmacokinetics, pharmacodynamics, lab
abnormalities, dose limiting toxicities
Initial Phase 1b Data Read-Out Expected in 1Q2024
a. Fast Track Designation granted for platinum resistant, ARID1A-mutated ovarian carcinoma,
12View entire presentation