Akouos IPO Presentation Deck
Internal Manufacturing Capabilities May Provide Competitive Advantage
42
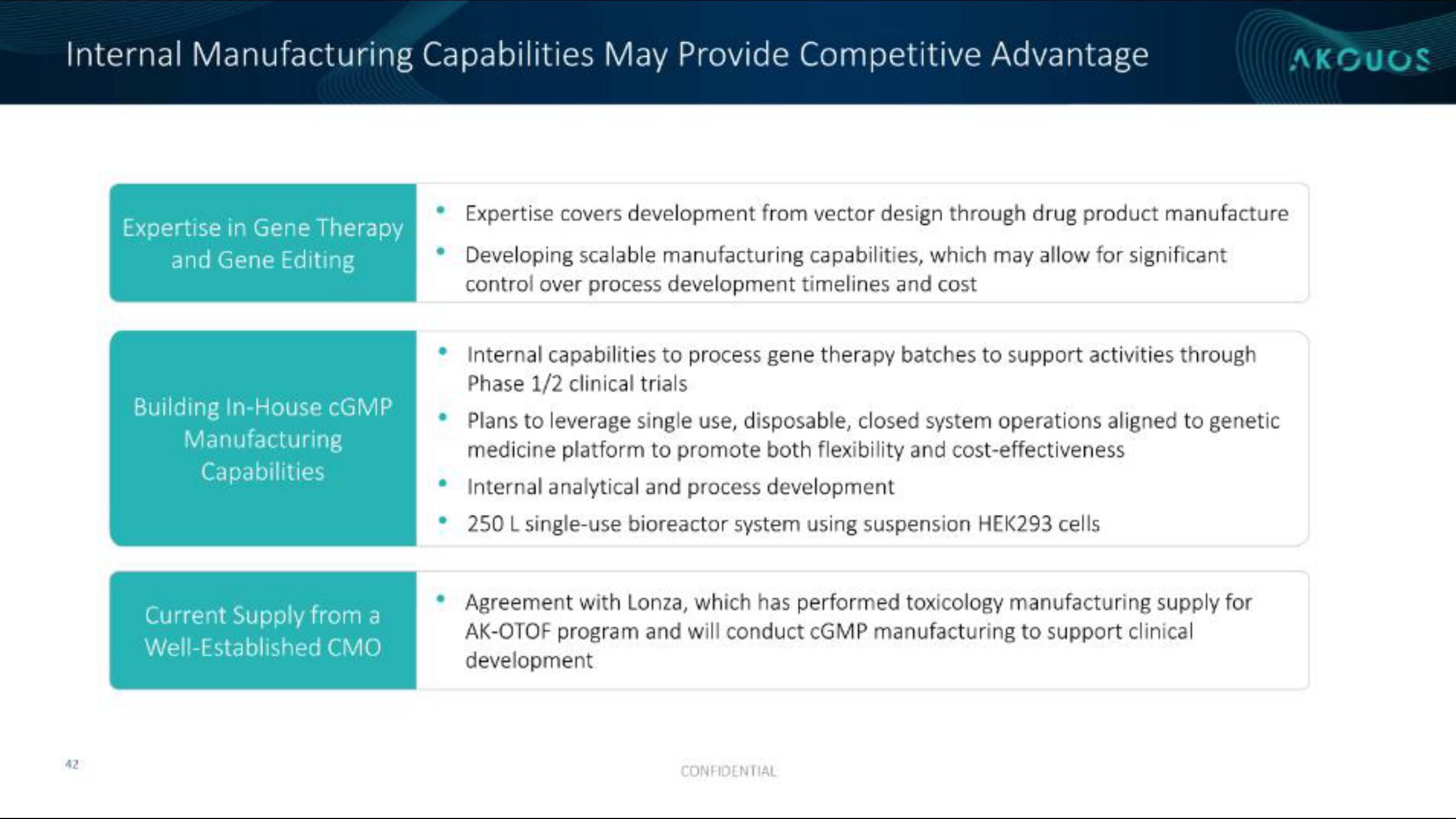
Expertise in Gene Therapy
and Gene Editing
Building In-House cGMP
Manufacturing
Capabilities
Current Supply from a
Well-Established CMO
Expertise covers development from vector design through drug product manufacture
Developing scalable manufacturing capabilities, which may allow for significant
control over process development timelines and cost
• Internal capabilities to process gene therapy batches to support activities through
Phase 1/2 clinical trials
Plans to leverage single use, disposable, closed system operations aligned to genetic
medicine platform to promote both flexibility and cost-effectiveness
Internal analytical and process development
• 250 L single-use bioreactor system using suspension HEK293 cells
Agreement with Lonza, which has performed toxicology manufacturing supply for
AK-OTOF program and will conduct CGMP manufacturing to support clinical
development
AKOUOS
CONFIDENTIALView entire presentation