Ocuphire Pharma Investor Update
RM
Percent of Subjects (%)
18
100%
80%
60%
40%
20%
0%
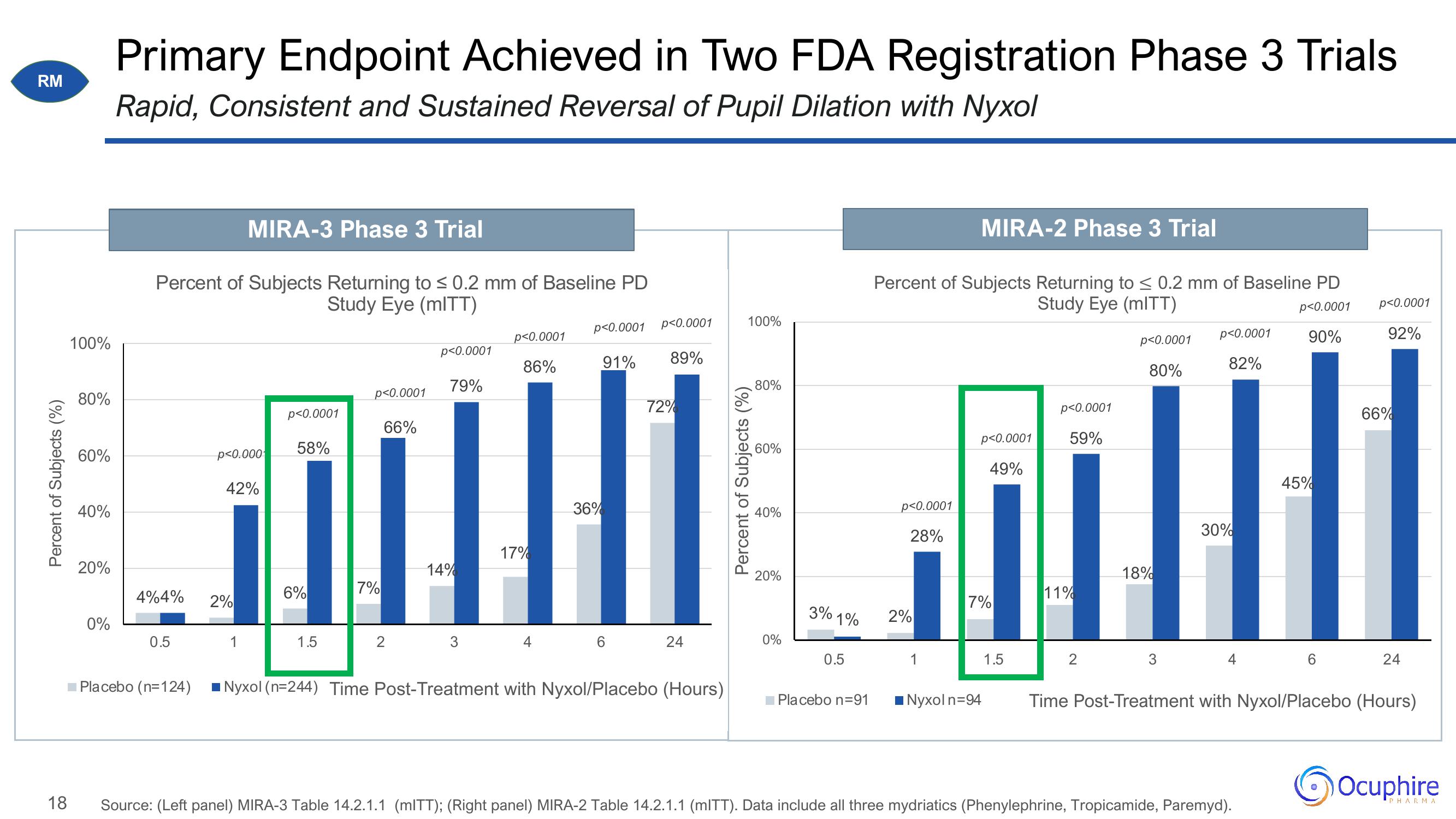
Primary Endpoint Achieved in Two FDA Registration Phase 3 Trials
Rapid, Consistent and Sustained Reversal of Pupil Dilation with Nyxol
Percent of Subjects Returning to ≤ 0.2 mm of Baseline PD
Study Eye (mITT)
4%4%
0.5
MIRA-3 Phase 3 Trial
p<0.000
42%
2%
1
p<0.0001
58%
6%
1.5
p<0.0001
7%
66%
2
p<0.0001
79%
14%
3
p<0.0001
86%
17%
4
p<0.0001 p<0.0001
91% 89%
36%
6
72%
24
Placebo (n=124) Nyxol (n=244) Time Post-Treatment with Nyxol/Placebo (Hours)
100%
Percent of Subjects (%)
80%
60%
40%
20%
0%
3% 1%
0.5
Placebo n=91
Percent of Subjects Returning to ≤ 0.2 mm of Baseline PD
Study Eye (mITT)
p<0.0001
90%
p<0.0001
28%
2%
1
MIRA-2 Phase 3 Trial
p<0.0001
49%
7%
■Nyxol n=94
1.5
p<0.0001
59%
11%
2
p<0.0001
80%
18%
3
p<0.0001
82%
30%
4
45%
Source: (Left panel) MIRA-3 Table 14.2.1.1 (mITT); (Right panel) MIRA-2 Table 14.2.1.1 (mITT). Data include all three mydriatics (Phenylephrine, Tropicamide, Paremyd).
6
p<0.0001
92%
66%
24
Time Post-Treatment with Nyxol/Placebo (Hours)
Ocuphire
PHARMAView entire presentation