AstraZeneca Results Presentation Deck
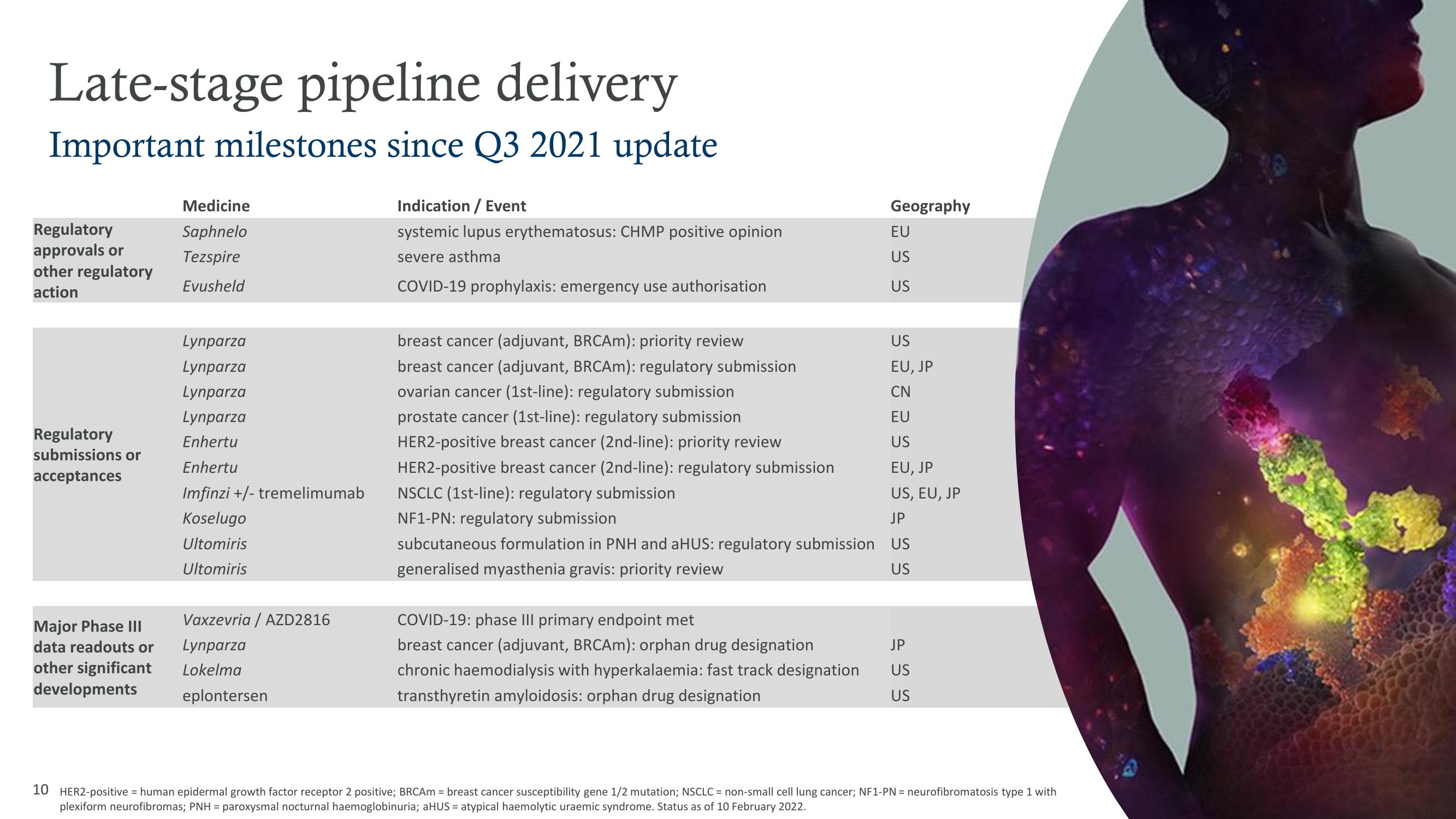
Late-stage pipeline delivery
Important milestones since Q3 2021 update
Regulatory
approvals or
other regulatory
action
Regulatory
submissions or
acceptances
Major Phase III
data readouts or
other significant
developments
Medicine
Saphnelo
Tezspire
Evusheld
Lynparza
Lynparza
Lynparza
Lynparza
Enhertu
Enhertu
Imfinzi +/- tremelimumab
Koselugo
Ultomiris
Ultomiris
Vaxzevria / AZD2816
Lynparza
Lokelma
eplontersen
Indication / Event
systemic lupus erythematosus: CHMP positive opinion
severe asthma
COVID-19 prophylaxis: emergency use authorisation
breast cancer (adjuvant, BRCAm): priority review
breast cancer (adjuvant, BRCAm): regulatory submission
ovarian cancer (1st-line): regulatory submission
prostate cancer (1st-line): regulatory submission
HER2-positive breast cancer (2nd-line): priority review
HER2-positive breast cancer (2nd-line): regulatory submission
NSCLC (1st-line): regulatory submission
NF1-PN: regulatory submission
Geography
COVID-19: phase III primary endpoint met
breast cancer (adjuvant, BRCAm): orphan drug designation
chronic haemodialysis with hyperkalaemia: fast track designation
transthyretin amyloidosis: orphan drug designation
EU
US
US
US
EU, JP
CN
EU
US
EU, JP
US, EU, JP
JP
subcutaneous formulation in PNH and aHUS: regulatory submission US
generalised myasthenia gravis: priority review
US
JP
US
US
10 HER2-positive = human epidermal growth factor receptor 2 positive; BRCAm = breast cancer susceptibility gene 1/2 mutation; NSCLC = non-small cell lung cancer; NF1-PN = neurofibromatosis type 1 with
plexiform neurofibromas; PNH = paroxysmal nocturnal haemoglobinuria; aHUS = atypical haemolytic uraemic syndrome. Status as of 10 February 2022.View entire presentation