Immix Biopharma Investor Presentation Deck
NXC-201 MoA: Next Generation CAR-T For Multiple Myeloma and AL Amyloidosis
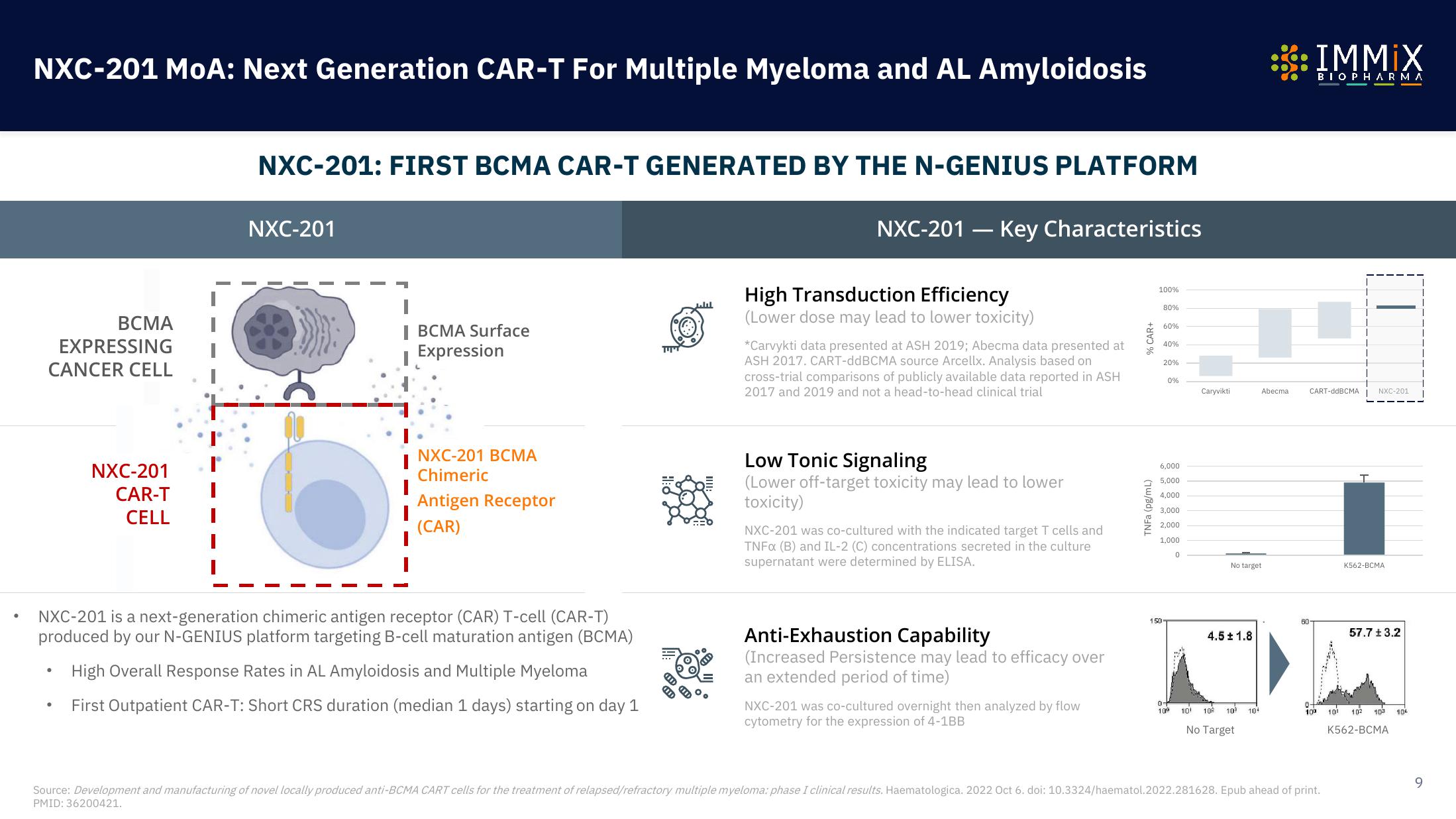
BCMA
EXPRESSING
CANCER CELL
NXC-201
CAR-T
CELL
●
NXC-201: FIRST BCMA CAR-T GENERATED BY THE N-GENIUS PLATFORM
NXC-201
BCMA Surface
Expression
NXC-201 BCMA
Chimeric
Antigen Receptor
(CAR)
NXC-201 is a next-generation chimeric antigen receptor (CAR) T-cell (CAR-T)
produced by our N-GENIUS platform targeting B-cell maturation antigen (BCMA)
High Overall Response Rates in AL Amyloidosis and Multiple Myeloma
First Outpatient CAR-T: Short CRS duration (median 1 days) starting on day 1
THPT
LID
لسل
NXC-201 - Key Characteristics
High Transduction Efficiency
(Lower dose may lead to lower toxicity)
*Carvykti data presented at ASH 2019; Abecma data presented at
ASH 2017. CART-ddBCMA source Arcellx. Analysis based on
cross-trial comparisons of publicly available data reported in ASH
2017 and 2019 and not a head-to-head clinical trial
Low Tonic Signaling
(Lower off-target toxicity may lead to lower
toxicity)
NXC-201 was co-cultured with the indicated target T cells and
TNFx (B) and IL-2 (C) concentrations secreted in the culture
supernatant were determined by ELISA.
Anti-Exhaustion Capability
(Increased Persistence may lead to efficacy over
an extended period of time)
NXC-201 was co-cultured overnight then analyzed by flow
cytometry for the expression of 4-1BB
% CAR+
TNFa (pg/mL)
100%
80%
60%
150
40%
20%
0%
6,000
5,000
4,000
3,000
2,000
1,000
0
Caryvikti
No target
4.5±1.8
10 10⁰ 10² 10³ 104
No Target
●●●
IMMIX
S BIOPHARMA
Abecma
CART-ddBCMA
100
Source: Development and manufacturing of novel locally produced anti-BCMA CART cells for the treatment of relapsed/refractory multiple myeloma: phase I clinical results. Haematologica. 2022 Oct 6. doi: 10.3324/haematol.2022.281628. Epub ahead of print.
PMID: 36200421.
101
T
NXC-201
K562-BCMA
57.7 ±3.2
103
K562-BCMA
104
9View entire presentation