Neumora Therapeutics IPO Presentation Deck
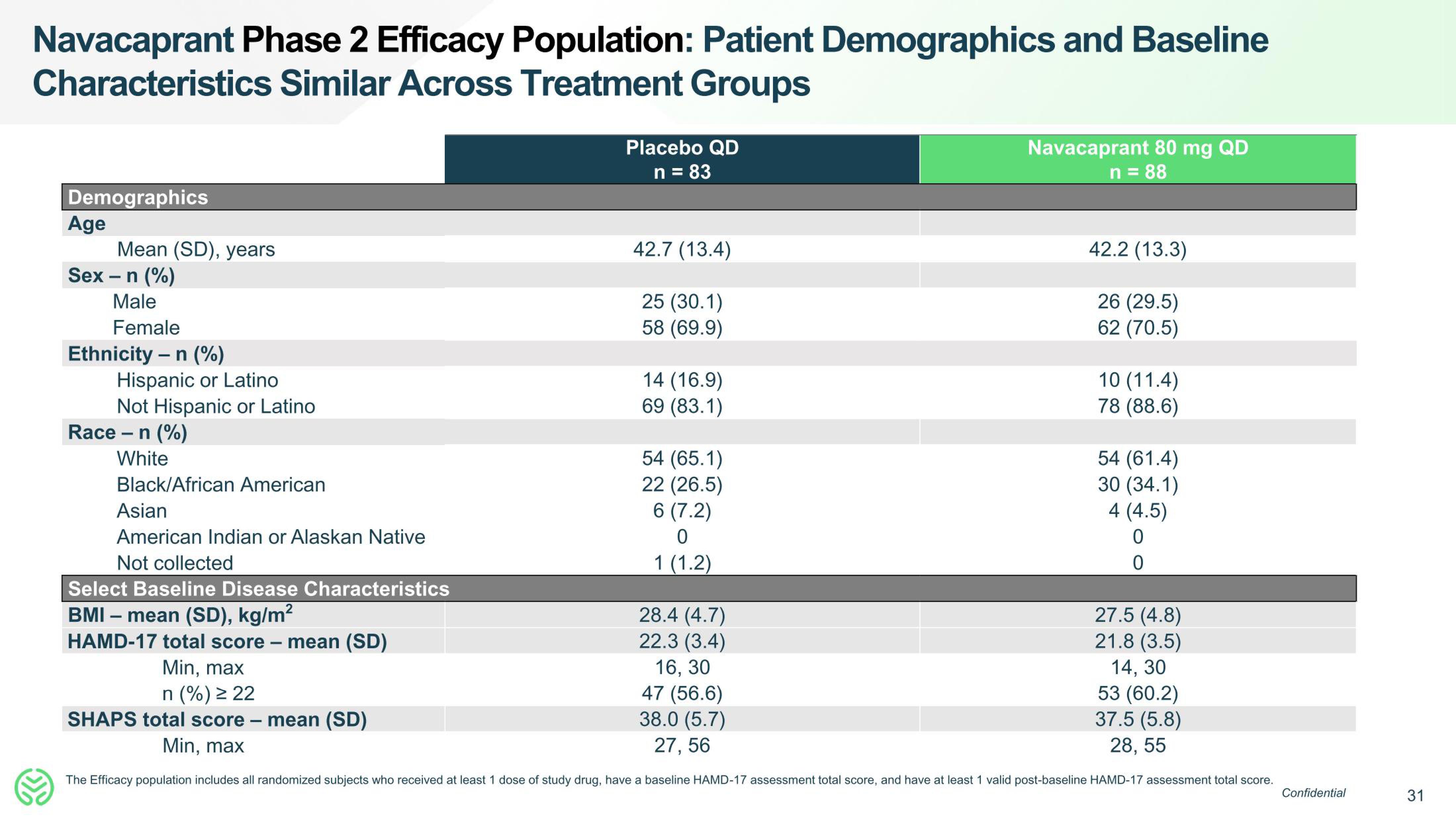
Navacaprant Phase 2 Efficacy Population: Patient Demographics and Baseline
Characteristics Similar Across Treatment Groups
Demographics
Age
Sex - n (%)
Male
Female
Ethnicity - n (%)
Mean (SD), years
Race
Hispanic or Latino
Not Hispanic or Latino
- n (%)
White
Black/African American
Asian
American Indian or Alaskan Native
Not collected
Select Baseline Disease Characteristics
BMI - mean (SD), kg/m²
HAMD-17 total score
-
mean (SD)
Placebo QD
n = 83
Min, max
n (%) ≥ 22
>
SHAPS total score - mean (SD)
Min, max
42.7 (13.4)
25 (30.1)
58 (69.9)
14 (16.9)
69 (83.1)
54 (65.1)
22
(26.5)
6 (7.2)
0
1 (1.2)
Navacaprant 80 mg QD
n = 88
42.2 (13.3)
26 (29.5)
62 (70.5)
10 (11.4)
78 (88.6)
28.4 (4.7)
22.3 (3.4)
16, 30
47 (56.6)
38.0 (5.7)
27, 56
27.5 (4.8)
21.8 (3.5)
14, 30
53 (60.2)
37.5 (5.8)
28, 55
The Efficacy population includes all randomized subjects who received at least 1 dose of study drug, have a baseline HAMD-17 assessment total score, and have at least 1 valid post-baseline HAMD-17 assessment total score.
54 (61.4)
30 (34.1)
4 (4.5)
0
0
||
Confidential
31View entire presentation